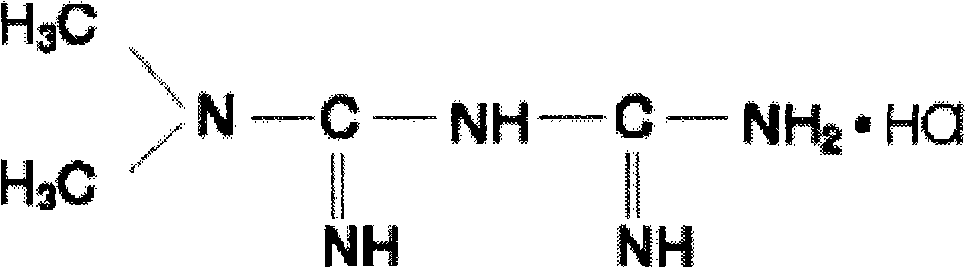Metformin controlled release tablet
A metformin and controlled-release tablet technology, which can be used in medical preparations with non-active ingredients, pill delivery, metabolic diseases, etc., and can solve problems such as increased osmotic pressure, increased incidence of adverse reactions, and increased metformin release.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Tablet Prescription:
[0060] Component
[0062] Tablet preparation method:
[0063] 1. Take metformin and pass through an 80-mesh sieve;
[0064] 2. Weigh metformin, polyoxyethylene, povidone k90, and sodium lauryl sulfate according to the prescription amount, mix well, add an appropriate amount of water-based soft material, pass through a 20-mesh sieve to granulate, and dry completely by blasting at 40°C;
[0065] 3. After the granules are completely dried, sieve the granules with a 20-mesh sieve, mix them evenly with the prescribed amount of magnesium stearate, and press them into tablets.
[0066] Coating solution prescription (1000ml coating solution dosage)
[0067] Cellulose acetate 30g
[0068] Macrogol 1500 4g
[0069] Triethyl citrate 3g
[0070] Acetone 900ml
[0071] water 100ml
[0072] Preparation method:
[0073] 1. Weigh 4g of polyethylene glycol 1500, add 100ml of water, soak to dissolve completely...
Embodiment 2
[0086] Tablet Prescription:
[0087] Component
[0088] Tablet cores were prepared according to the tablet core preparation method in Example 1 according to the prescription quantity of tablet cores, and the same coating solution as in Example 1 was used, and the same coating and punching method as in Example 1 was used to prepare metformin controlled release in this example. finished piece.
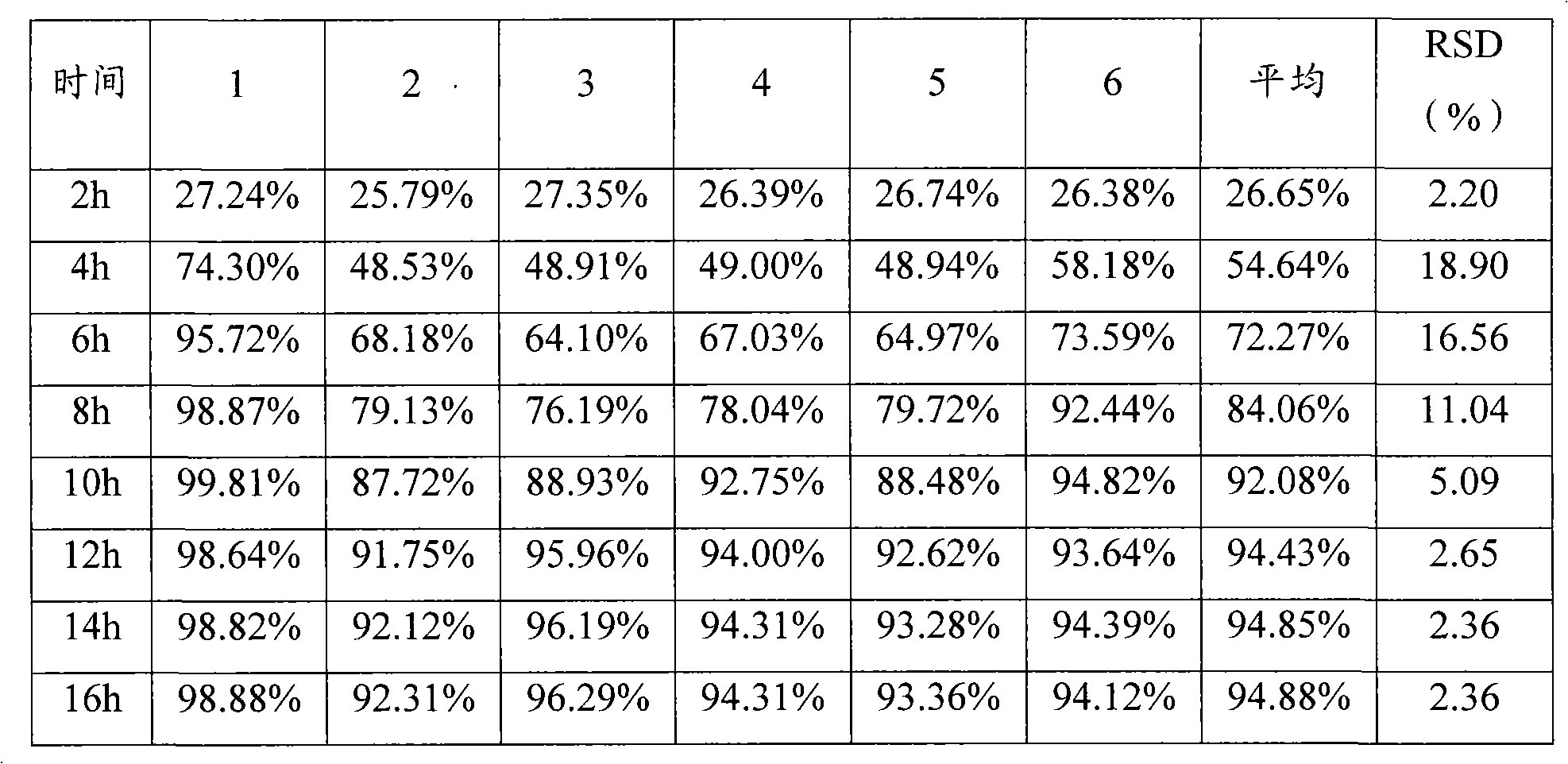
[0089] Release test results
[0090] time
[0091] 14h
Embodiment 3
[0093] Tablet Prescription:
[0094] Component
Content mg / tablet
percentage
1000
83.5%
Povidone K90
62.5
5%
12.5
1%
Polyoxyethylene N80
62.5
5%
68.75
5.5%
[0095] Tablet cores were prepared according to the tablet core preparation method in Example 1 according to the prescription quantity of tablet cores, and the same coating solution as in Example 1 was used, and the same coating and punching method as in Example 1 was used to prepare metformin controlled release in this example. finished piece.
[0096] Release test results
[0097] time
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com