Fenofibrate osmotic pump controlled release preparation and preparation method thereof
A technology of fenofibrate and controlled-release preparations, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc., to achieve the effect of reducing the number of medications, reducing side effects, and stabilizing and lasting blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
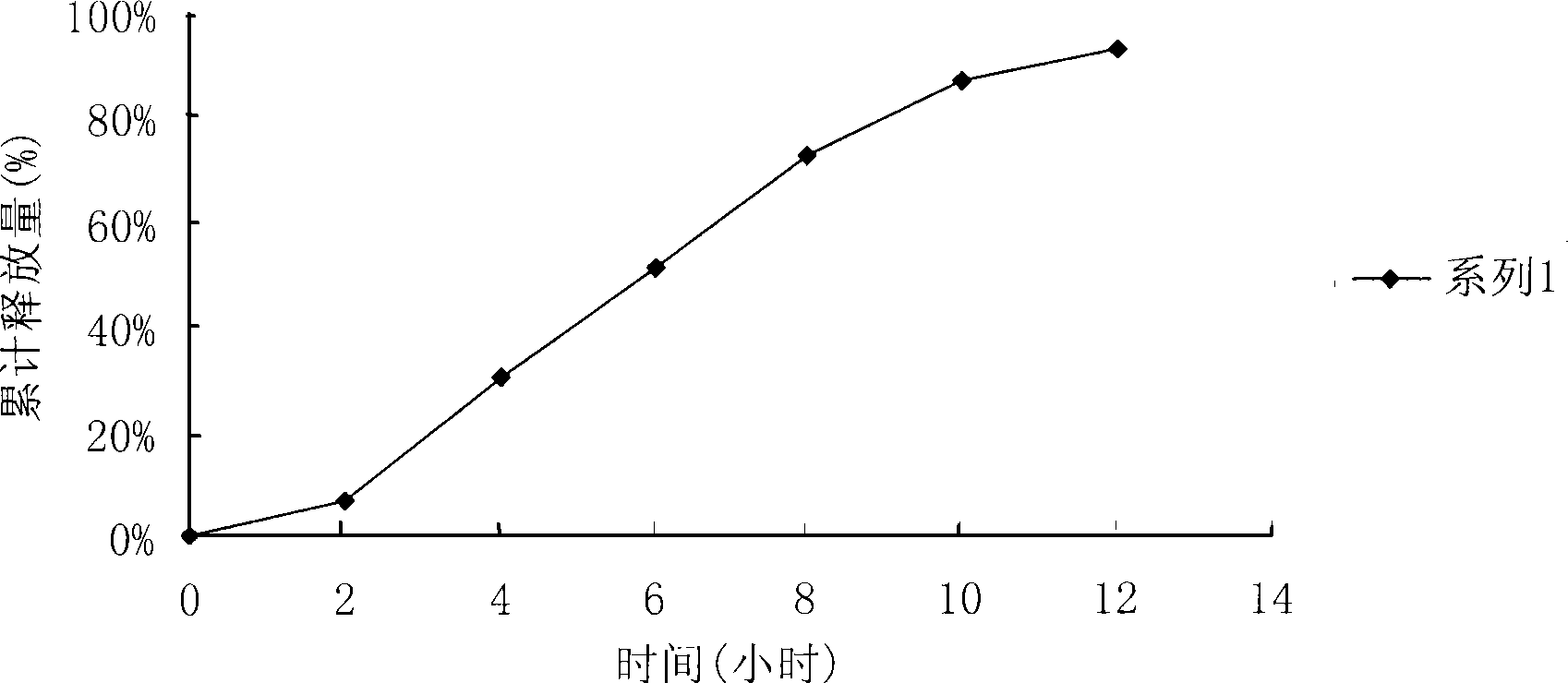
Image
Examples
Embodiment 1
[0032] Example 1 Fenofibrate double-layer osmotic pump
[0033] Tablet core prescription (by weight)
[0034] Drug layer:
[0035] Fenofibrate 250mg
[0036] NaCl 65mg
[0037] Polyoxyethylene WSR205 200mg
[0038] Magnesium stearate amount
[0039] Booster layer:
[0040] Polyoxyethylene WSR303 145mg
[0041] Lactose 10mg
[0042] Iron oxide red 1mg
[0043] 95% ethanol appropriate amount
[0044] Semi-permeable membrane prescription (by weight)
[0045] Cellulose acetate 18g
[0046] Polyethylene glycol 2000 4g
[0047] Solvent formulation for dissolving coating material (volume calculation)
[0048] Acetone 500ml
[0049] water 50ml
[0050] Preparation Process:
[0051] Mix the prescribed amount of medicine with NaCl and WSR205 that have passed through an 80-mesh sieve, use 95% ethanol solution as a binder to make soft materials, granulate with a 20-mesh sieve, dry the wet granules, and use a 18-mesh sieve to granulate. After adding lubricant and mixing eve...
Embodiment 2
[0052] Example 2 Fenofibrate double-layer osmotic pump
[0053] Tablet core prescription (by weight)
[0054] Drug layer:
[0055] Fenofibrate 250mg
[0056] Polyoxyethylene PEO 1105 100mg
[0057] Polyoxyethylene PEO N80 100mg
[0058] Xylitol 100mg
[0059] Booster layer:
[0060] Sodium Alginate 140mg
[0061] Sodium Bicarbonate 30mg
[0062] Iron oxide red 1mg
[0063] Magnesium stearate amount
[0064] 10% PVP alcohol solution
[0065] Semi-permeable membrane prescription (by weight)
[0066] Cellulose acetate 15g
[0067] PEG4000 5g
[0068] Solvent ratio of coating film material (calculated by volume)
[0069] Acetone 500ml
[0070] water 30ml
[0071] Mix the prescribed amount of medicine with NaCl, PEO 1105 and PEO N80 that have passed through a 80-mesh sieve, and use 10% PVP alcohol solution as a binder to make a soft material, granulate with a 20-mesh sieve, and dry the wet granules with an 18-mesh sieve. The whole dry granules are mixed with lubricant...
Embodiment 3
[0072] Example 3 Fenofibrate double-layer osmotic pump
[0073] Tablet core prescription (by weight)
[0074] Drug-containing layer:
[0075] Fenofibrate 250mg
[0076] Polyoxyethylene PEO N12K 175mg
[0077] Glucose 125mg
[0078] Booster layer:
[0079] Polyoxyethylene WSR 303 145mg
[0080] NaCl 5mg
[0081] Iron oxide red amount
[0082] Magnesium stearate amount
[0083] 1% HPMC alcohol solution
[0084] Semi-permeable membrane prescription (by weight)
[0085] Cellulose acetate 25g
[0086] PEG1500 7g
[0087] Solvent ratio of coating film material (calculated by volume)
[0088] Acetone 1000ml
[0089] water 80ml
[0090] Mix the prescribed amount of medicine with glucose and PEO N12K that have passed through an 80-mesh sieve, use 10% PVP ethanol solution as a binder to make a soft material, granulate with a 20-mesh sieve, dry the wet granules, and use an 18-mesh sieve to granulate. After the dry granules are mixed with lubricant, the tablet core is obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com