Ranitidine bismuth citrate intra-gastric floating sustained-release tablet and preparation method thereof
A technology of bismuth citrate ranitidine and gastric flotation, which is applied in the directions of pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of patients taking medicines frequently, poor compliance, etc. , to achieve the effect of lasting blood drug concentration, good stability, and reducing the number of times of medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of embodiment 1RBC gastric floating tablet
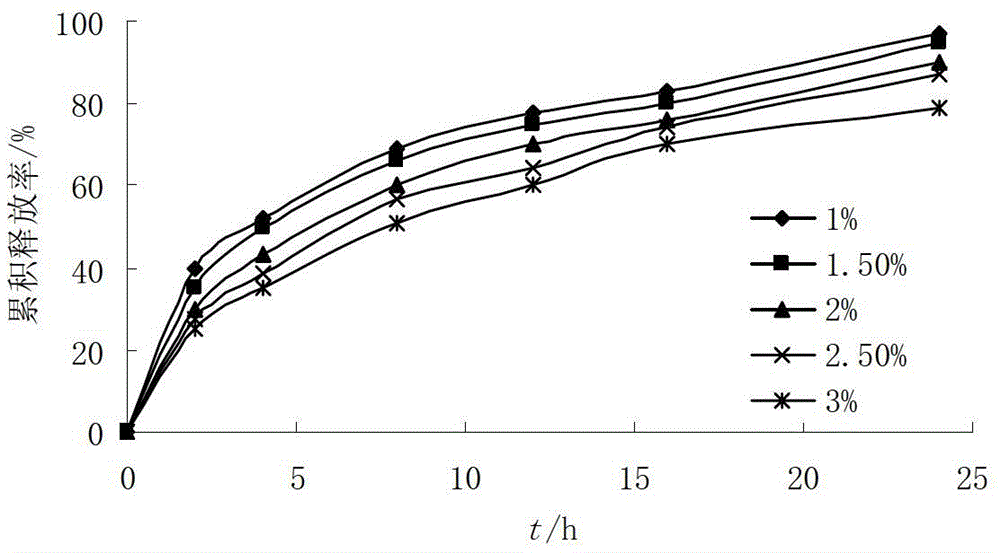
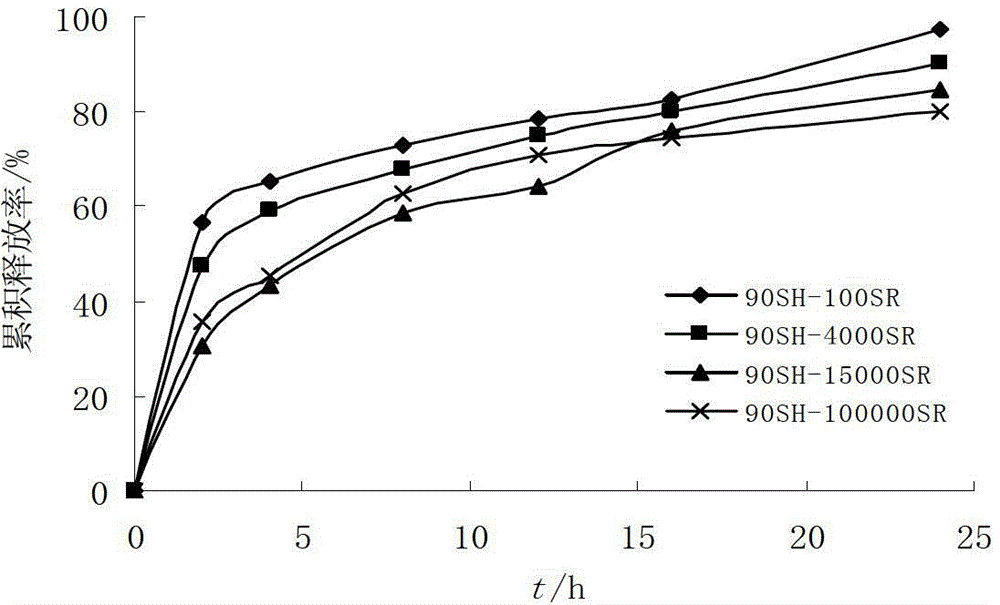
[0045] Tablet core formula: RBC raw material 228g, stearyl alcohol 240g, HPMC 15000SR 60g, HPMC 100000SR 30g, NaHCO 3 12g, micronized silica gel 2g, magnesium stearate 2g, absolute ethanol 10g.
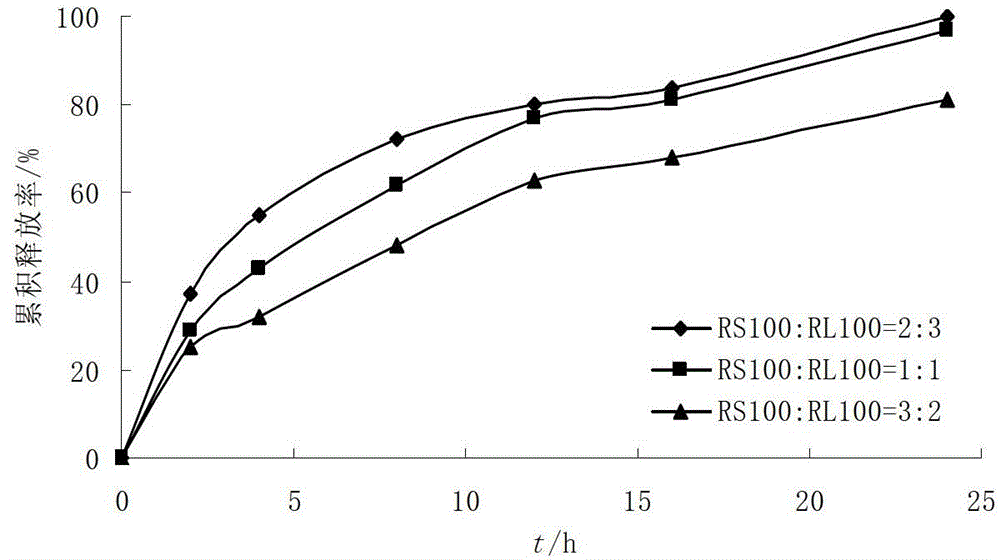
[0046] Coating film coating material: Eudragit RS100, RL100 30g each, triethyl citrate 6g, talcum powder 15g, ethanol 919g.
[0047] Preparation:
[0048] Pass the raw material and auxiliary materials through a 100-mesh sieve for later use; weigh the prescribed amount of raw materials and auxiliary materials in equal amounts and mix them uniformly, add an appropriate amount of binder, and make a soft material. Granulate through a 30-mesh sieve, and dry in an oven at 45°C for 2-3 hours. Sieve through a 24-mesh sieve, add a lubricant (the same amount of micro-powder silica gel and magnesium stearate) and mix well; control the tablet hardness at 40-50N, punch the tablet with a 12mm shallow concave; that is, the table...
Embodiment 2
[0051] The preparation of embodiment 2RBC gastric floating tablet
[0052] Tablet core formula: RBC raw material 228g, stearyl alcohol 240g, HPMC 15000SR 40g, HPMC 100000SR 20g, NaHCO 3 12g, magnesium stearate 4g, absolute ethanol 10g.
[0053] Coating film coating material: Eudragit RS100, RL100 30g each, triethyl citrate 6g, talcum powder 15g, ethanol 919g.
[0054] Preparation:
[0055] Pass the raw material and auxiliary materials through an 80-mesh sieve for later use; weigh the prescribed amount of raw materials and auxiliary materials in equal amounts and mix them uniformly, add an appropriate amount of binder, and make a soft material. Pass through a 30-mesh sieve to granulate, and dry in an oven at 50°C for 2 hours. Sieve with a 24-mesh sieve, add a lubricant and mix evenly; control the tablet hardness at 40-50N, punch the tablet with a 12mm shallow concave; that is, the tablet core.
[0056] Weigh the coating material in proportion and dissolve it in 500g of eth...
Embodiment 3
[0058] The preparation of embodiment 3RBC gastric floating tablet
[0059] Tablet core formula: RBC raw material 228g, stearyl alcohol 240g, HPMC 15000SR 80g, HPMC 100000SR 40g, NaHCO 3 12g, magnesium stearate 4g, absolute ethanol 10g.
[0060] Coating film coating material: Eudragit RS100, RL100 30g each, triethyl citrate 6g, talcum powder 15g, ethanol 919g.
[0061] Preparation:
[0062] Pass the raw material and auxiliary materials through a 120-mesh sieve for later use; weigh the prescribed amount of raw materials and auxiliary materials in equal amounts and mix them uniformly, add an appropriate amount of binder, and make a soft material. Granulate through a 30-mesh sieve, and dry in an oven at 40°C for 3 hours. Sieve with a 24-mesh sieve, add a lubricant and mix evenly; control the tablet hardness at 40-50N, punch the tablet with a 12mm shallow concave; that is, the tablet core.
[0063] Weigh the coating material in proportion and dissolve it in 500g of ethanol, ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com