Preparation method of glipizide osmotic pump controlled release tablet
A glipizide tablet and osmotic pump controlled-release technology, which is applied in the field of preparation of glipizide osmotic pump controlled-release tablets, can solve the problems of difficult drying, large amount of excipients, and long time consumption, and achieve easy-to-accept therapeutic effects, The effect of improving the dissolution rate and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
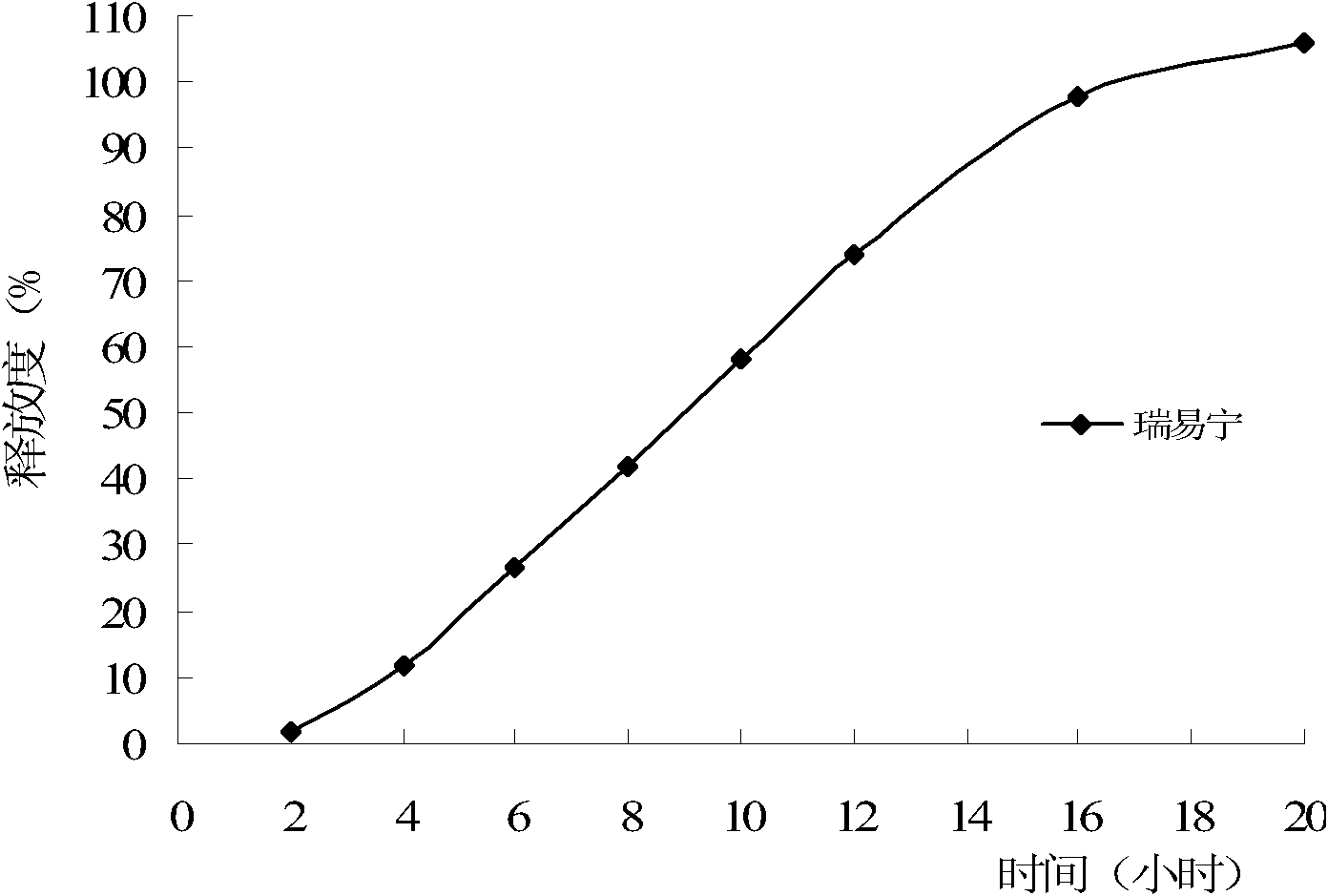
Image
Examples
Embodiment 1
[0040] A preparation method of glipizide osmotic pump controlled-release tablet:
[0041] A: Preparation of solid dispersion
[0042] prescription:
[0043]
[0044] Get acetone (China Petroleum and Chemical Corporation, the same below) and put it in a container, add povidone K30 (US ISP company, the same below) and sodium lauryl sulfate (Germany Congis company, the same below) to make it dissolve or Evenly disperse, heat the acetone solution at 35°C, add glipizide (Weihai Diso Pharmaceutical Co., Ltd., the same below) while stirring, keep stirring to make it disperse evenly, dry, make the loss on drying within 2%, and pass 24 Mesh sieve.
[0045] B: Preparation of tablet core
[0046] prescription:
[0047]
[0048] Get glipizide solid dispersion, meglumine (Shanghai Pharmaceutical Group Co., Ltd., the same below), povidone K30, hypromellose E50 (U.S. Dow company, the same below), sodium chloride (Tianjin Haiguang Pharmaceutical Co., Ltd. Industry Co., Ltd., the sa...
Embodiment 2
[0057] A preparation method of glipizide osmotic pump controlled-release tablet:
[0058] A: Preparation of solid dispersion
[0059] prescription:
[0060]
[0061] Take isopropanol and put it in a container, add Tween 80 (Shanghai Shengyu Chemical Co., Ltd., the same below), lactose (Shanghai Huamao Pharmaceutical Co., Ltd., the same below) and hypromellose E3 (U.S. Dow Company, The same below) to make it dissolve or disperse evenly, keep the isopropanol solution at 35°C, add glipizide while stirring, keep stirring to make it disperse evenly, dry, make the loss on drying within 2%, pass through a 24 mesh sieve .
[0062] B: Preparation of tablet core
[0063] prescription:
[0064]
[0065] Take glipizide solid dispersion, meglumine, povidone K30, polyethylene glycol-8000 (Xi'an Hui'an Cellulose Chemical Co., Ltd.), sodium chloride and mix evenly, then add magnesium stearate and mix evenly. Tablet, the hardness is controlled at 100N.
[0066] C: Coating of semi-p...
Embodiment 3
[0074] A preparation method of glipizide osmotic pump controlled-release tablet:
[0075] A: Preparation of solid dispersion
[0076] prescription:
[0077]
[0078] Take water and put it in a container, add meglumine, hypromellose E15 (Dow Company of the United States, the same below) and mannitol (Roquette Company of France) to dissolve or disperse evenly, and use it as the water phase for future use. Take 95% ethanol and put it in another container, add glipizide to make it disperse, as the ethanol phase. With continuous stirring, the aqueous phase was slowly added to the ethanol phase. Continue to stir for about 30 minutes after the addition, dry to make the loss on drying within 2%, and pass through a 24-mesh sieve.
[0079] B: Preparation of tablet core
[0080] prescription:
[0081]
[0082] Take the above glipizide solid dispersion, poloxamer (Germany BASF company), hyprolose E50, polyoxyethylene N80 (U.S. Dow company), sodium chloride and magnesium stearat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com