Metformin hydrochloride controlled-release tablet and preparation method thereof
A technology for metformin hydrochloride and sustained-release tablets, which is used in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. and other problems, to achieve the effect of large production capacity, low production cost and small weight gain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
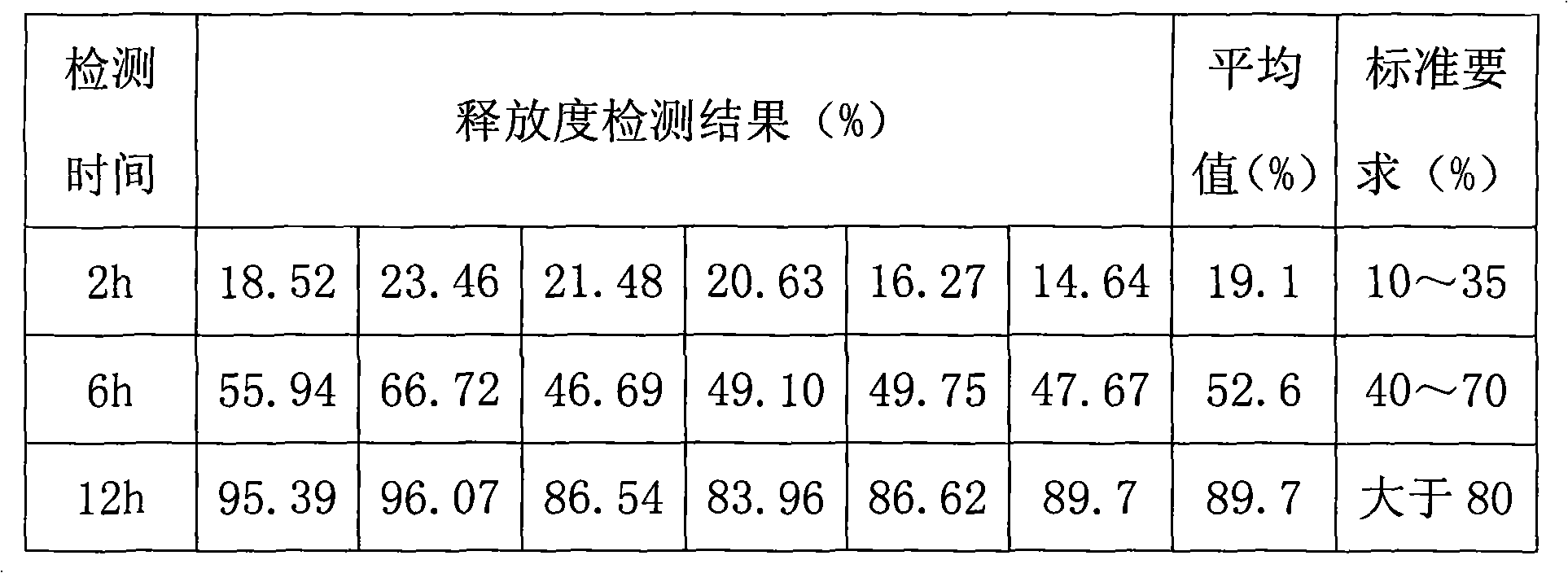
[0021] Embodiment: a kind of metformin hydrochloride sustained-release tablet, its preparation method is: at first metformin hydrochloride raw material and filler, binding agent are pulverized respectively, cross 120 mesh sieves; Add the agent into the wet granulator, stir evenly at a high speed, add the binder, continue to stir and mix, and make a soft material, then granulate, dry, granulate, add lubricant and press to get the plain tablet; the plain tablet is qualified Finally, pass the coating solution through a 100-200 mesh sieve, preheat the plain tablet to 40°C-50°C and start spraying, set the flow rate at 200-300ml / min, and control the speed at 4-6 rpm. The air temperature is 50°C-60°C, and the outlet air temperature is 40°C-50°C. Under the condition that the plain sheet does not stick to the pan, it is sprayed and dried to obtain the finished product.
example 1
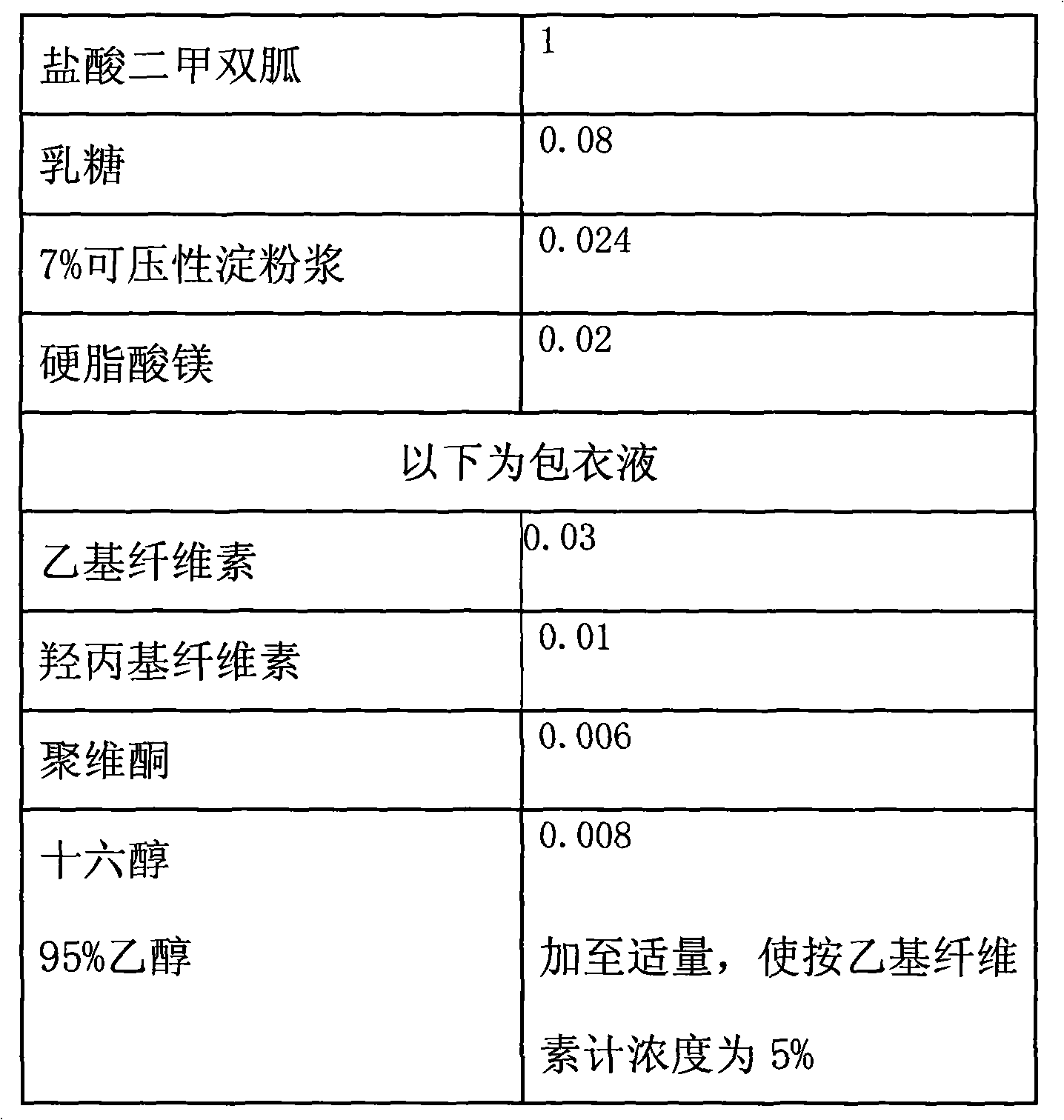
[0022] Example 1: The weight ratio of each component is shown in Table 1:
[0023] Table 1
[0024]
[0025]Preparation steps: according to the above proportions: firstly crush metformin hydrochloride, compressible starch, and lactose respectively, pass through a 120 mesh sieve, weigh them according to the prescription amount, and set aside; take ethyl cellulose and appropriate amount of 95% ethanol (refrigerated overnight) Finally, make up 95% ethanol according to the above-mentioned proportion to make a 5% coating liquid, for subsequent use;
[0026] Take compressible starch, prepare 7% starch slurry, and set aside;
[0027] Take metformin hydrochloride and lactose powder, mix them uniformly according to the principle of equal amount addition, and make soft material with 7% starch slurry.
[0028] Granulate with a 14-mesh sieve, dry at 55-60°C, and control the water content to less than 3%.
[0029] After sizing with a 16-mesh sieve, add the prescribed amount of magnes...
example 2
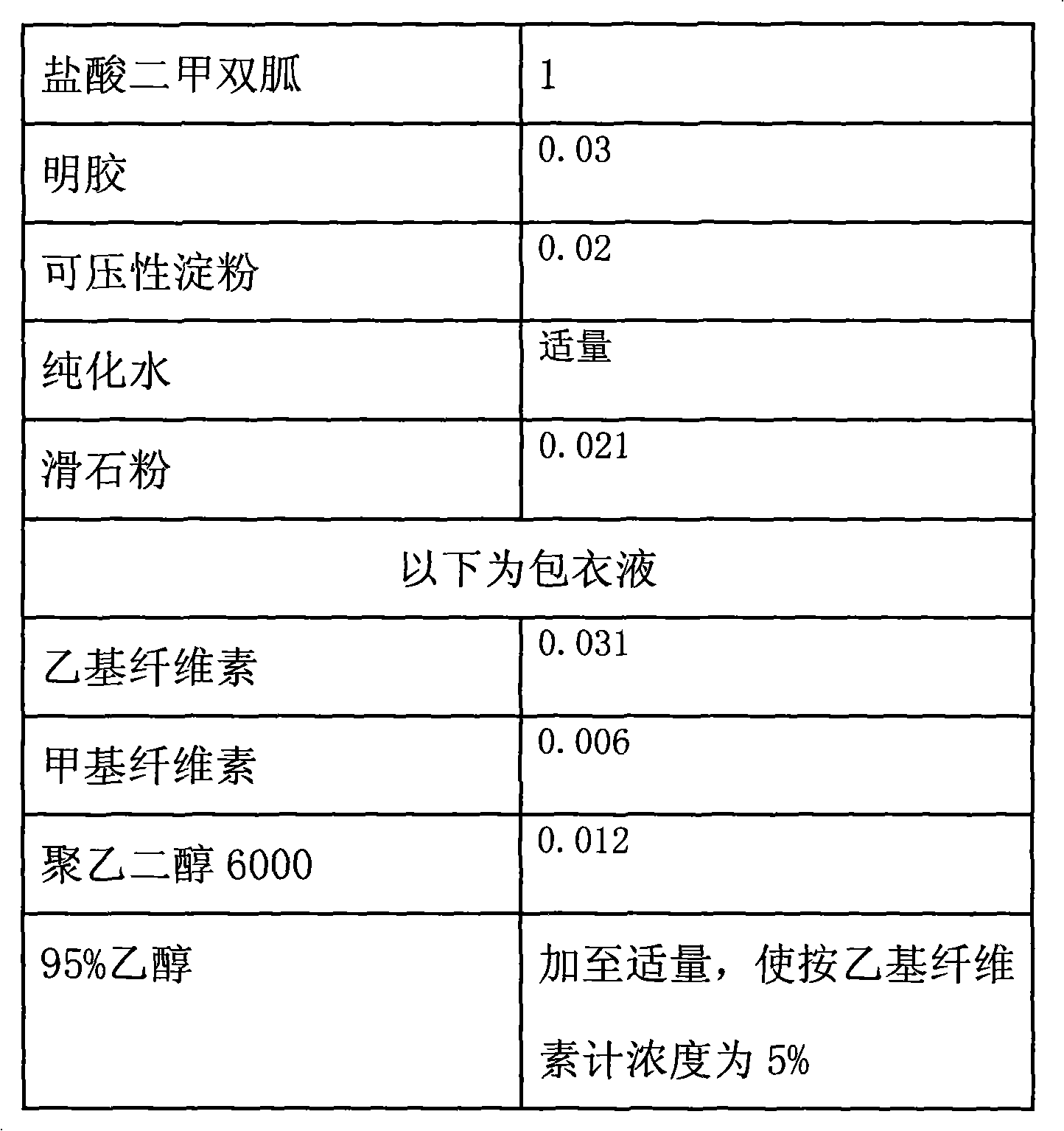
[0034] Example 2: The weight ratio of each component is shown in Table 3:
[0035] table 3
[0036]
[0037] Preparation steps: according to the above ratio: first, crush metformin hydrochloride, compressible starch, and gelatin, pass through a 120-mesh sieve, weigh them according to the prescription amount, and set aside; take ethyl cellulose, and an appropriate amount of 95% ethanol (refrigerated overnight) Finally, make up 95% ethanol according to the above-mentioned proportion to make a 5% coating liquid, for subsequent use;
[0038] Take metformin hydrochloride, gelatin powder, compressible starch and mix uniformly according to the principle of increasing in equal amounts, and make a soft material with an appropriate amount of purified water.
[0039] Granulate with a 14-mesh sieve, dry at 55-60°C, and control the water content to less than 3%.
[0040] After sizing with a 16-mesh sieve, add the prescribed amount of talcum powder, mix evenly, and press into tablets w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com