Method for building model for collectively evaluating bioavailability and toxicity of cadmium accumulation in food for human body
A bioavailability and model establishment technology, applied in the field of model establishment for joint evaluation of food cadmium accumulation on human bioavailability and toxicity, can solve the problems of bias, inaccuracy, and complicated operation in toxicity evaluation, and it is easy to achieve the test conditions. The effect of control, less pollution and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
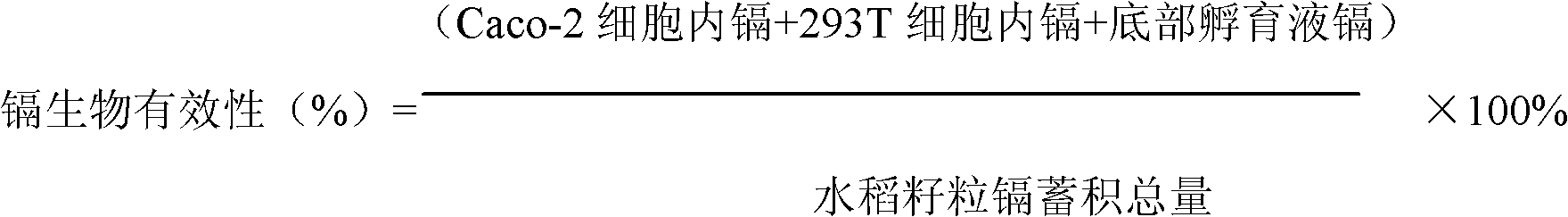
[0024] Example 1: Bioavailability and toxicity of rice cadmium accumulation to human body:
[0025] (1) Collect rice grains, grind them, pass through pepsin digestion solution with pH 2.0, digest in 37°C constant temperature water bath for 1 hour; trypsin-bile extract digestion liquid with pH 7.0, digest in 37°C constant temperature water bath 2h; centrifuge, collect the supernatant, and autoclave the supernatant;
[0026] (2) Evenly mix the supernatant with the culture solution (without cadmium) to make a mixed solution, draw 2.5mL of the mixed solution and add it to the Caco-2 cell layer of the established joint culture model, and at the same time, draw the isotonic culture medium Add 2.5mL of solution to the 293T cell layer, and continue to culture in a carbon dioxide incubator at 37°C for 24 hours; if the food cadmium accumulation is low, a chronic toxicity test can be carried out, and the longest culture can be continued for 10 days;
[0027] (3) Collect Caco-2 cells and...
Embodiment 2
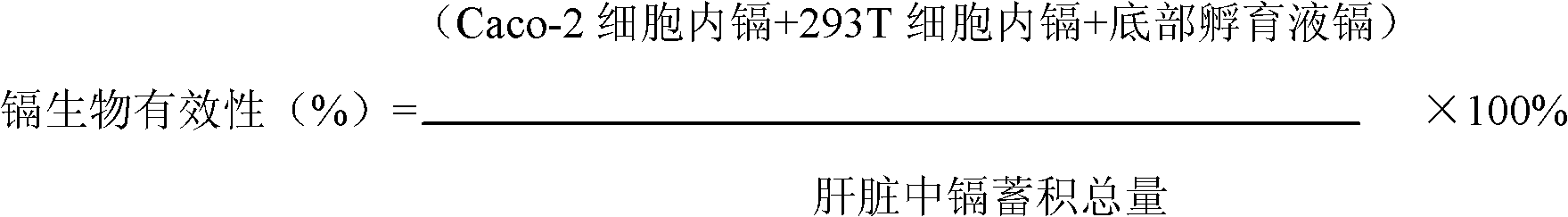
[0030] Example 2: Bioavailability and toxicity of animal liver cadmium accumulation to human body:
[0031] (1) Cook the animal liver, grind it, pass through the pepsin digestion solution with pH 2.0, and digest it in a constant temperature water bath at 37°C for 1 hour; trypsin-bile extract digestion liquid at pH 7.0, and digest it in a constant temperature water bath at 37°C Digest for 2 hours; centrifuge, collect the supernatant, and autoclave the supernatant;
[0032] (2) Evenly mix the supernatant with the culture solution (without cadmium) to make a mixed solution, draw 2.5mL of the mixed solution and add it to the Caco-2 cell layer of the established joint culture model, and at the same time, draw the isotonic culture medium Add 2.5mL of solution to the 293T cell layer, and continue to culture in a carbon dioxide incubator at 37°C for 24 hours; if the food cadmium accumulation is low, a chronic toxicity test can be carried out, and the longest culture can be continued f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Transmittance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com