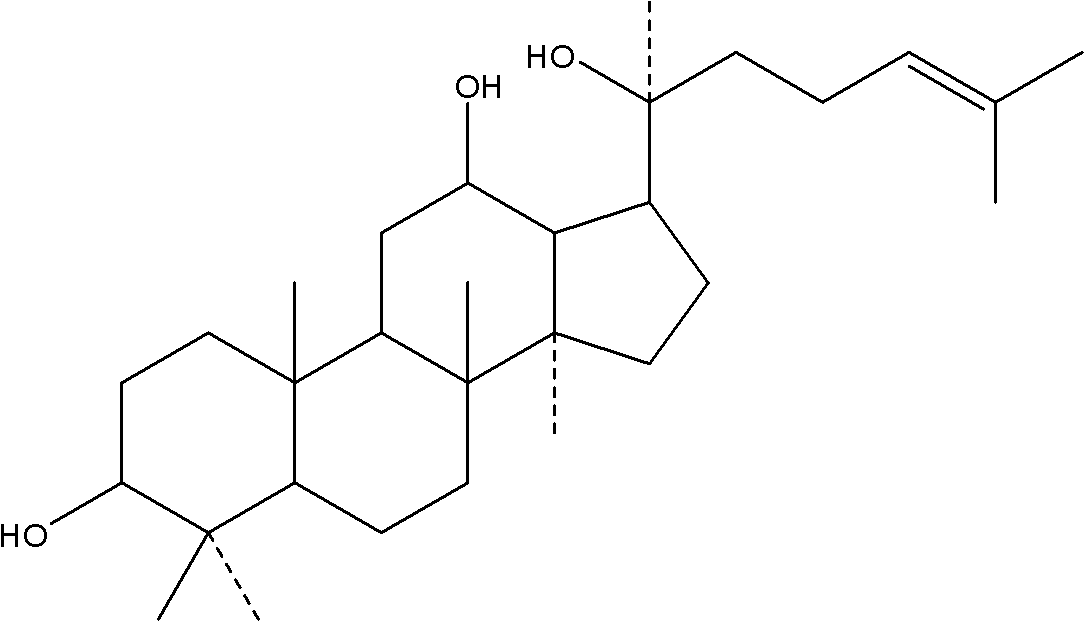
Preparation, medical application and composition of 20S-protopanaxadiol beta-cyclodextrin inclusion compound
A technology of cyclodextrin inclusion compound and protopanaxadiol, which is applied in the fields of pharmacy and food, and can solve problems such as difficulty in preparation, affecting drug stability and safety, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Preparation of β-cyclodextrin inclusion compound of 20S-protopanaxadiol of the present invention
[0046] A) Take 1 g of 20S-protopanaxadiol, add 1 g of β-cyclodextrin, add 6 ml of water, and grind for 3 hours. The measured inclusion rate is 84.1%, and the yield of 20S-protopanaxadiol inclusion compound is 98.2%. Before and after inclusion, the composition did not change.
[0047] B) Take 1g of 20S-protopanaxadiol, add 1g of β-cyclodextrin, add 6ml of absolute ethanol, ultrasonic power is 200W, ultrasonic time is 30min, and temperature is 30°C. Under this condition, the clathrate rate was 89.2%, and the clathrate yield was 91.2%.
[0048] C) Take 1 g of β-cyclodextrin, add 6 ml of water to dissolve at 60°C, and continuously add 20S-protopanaxadiol 1g of absolute ethanol solution to it under stirring, the inclusion time is 3 hours, and the inclusion temperature is 60°C. The clathrate rate can reach 94%, and the clathrate yield is 95.5%.
Embodiment 2
[0049] Example 2: Qualitative and quantitative analysis of the β-cyclodextrin inclusion compound of 20S-protopanaxadiol of the present invention
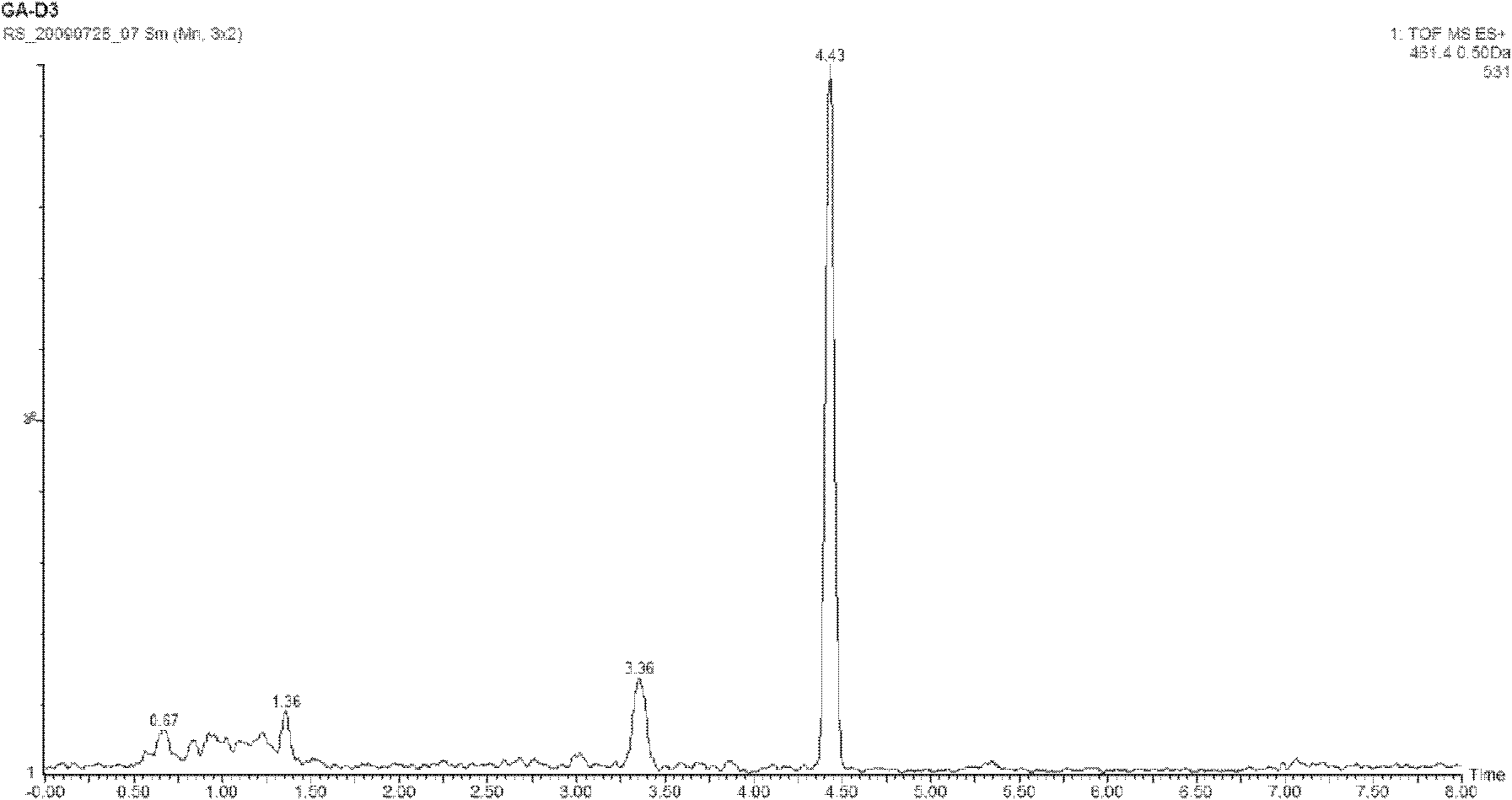
[0050] Chromatographic conditions and system suitability test used octadecyl bonded silica gel as filler, acetonitrile-water (85:15V / V), column temperature 30°C, flow rate 1.0ml / min. The number of theoretical plates calculated by 20S-protopanaxadiol should not be less than 2000. The separation between 20S-protopanaxadiol and adjacent peaks is greater than 1.5.
[0051] Detector: ultraviolet detection, the detection wavelength is 203nm; with mass spectrometry (MS) detector (461.4[M+H] + ) detection; time-of-flight mass spectrometry (TOF-MS) detector (461.3995[M+H] + ) detection; ultraviolet-visible diode array detector 190-203nm detection; circular dichroism (CD) detector 190-210nm detection; evaporative light scattering detector.
[0052] Configuration of the reference substance solution: Take 20S-protopanaxadiol chemical referenc...
Embodiment 3
[0055] Embodiment 3: the preparation of pharmaceutical composition
[0056] Any one of the compounds described in the present invention and a pharmaceutically acceptable carrier, or compatible with other drugs, are mixed with conventional pharmacy techniques to prepare tablets, capsules, granules, oral liquids, injections and other preparations.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com