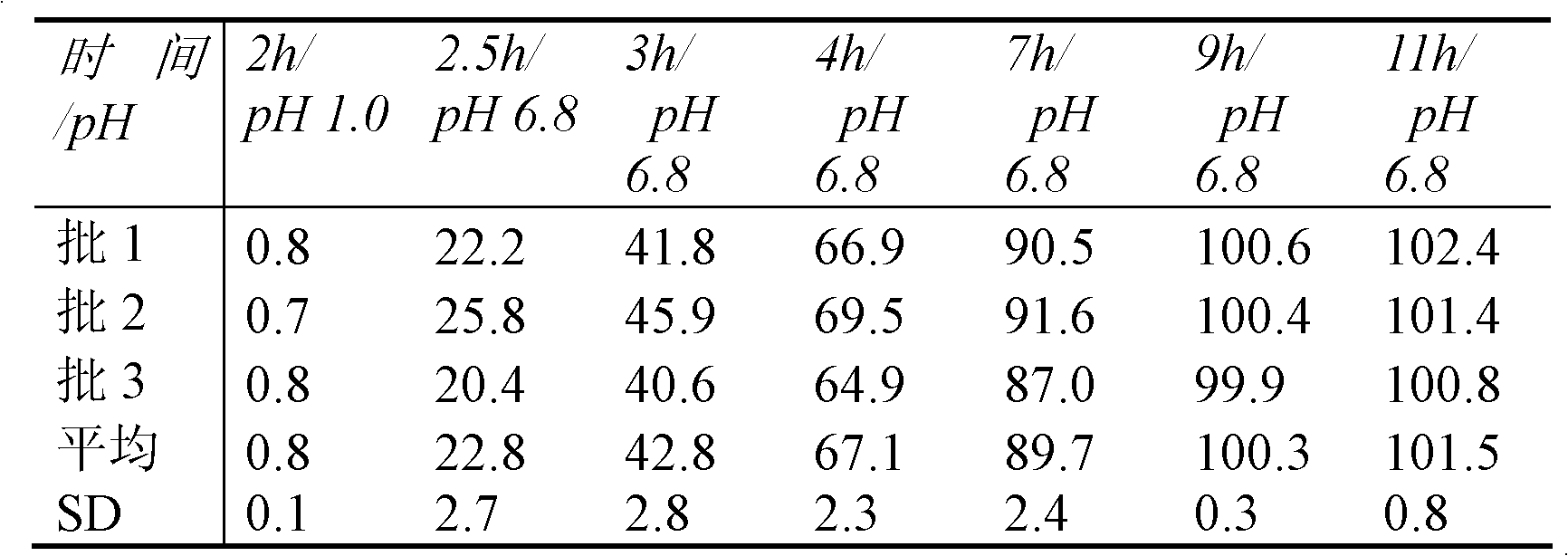Drug sustained and controlled release microparticle preparation for treating intestinal diseases, and preparation method thereof
A technology for intestinal diseases and drugs, which is applied in the field of sustained and controlled release pellets or granule preparations and the preparation of the above slow and controlled release pellets or granule preparations. Compliance, reducing the number of doses taken, and ensuring the effect of uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
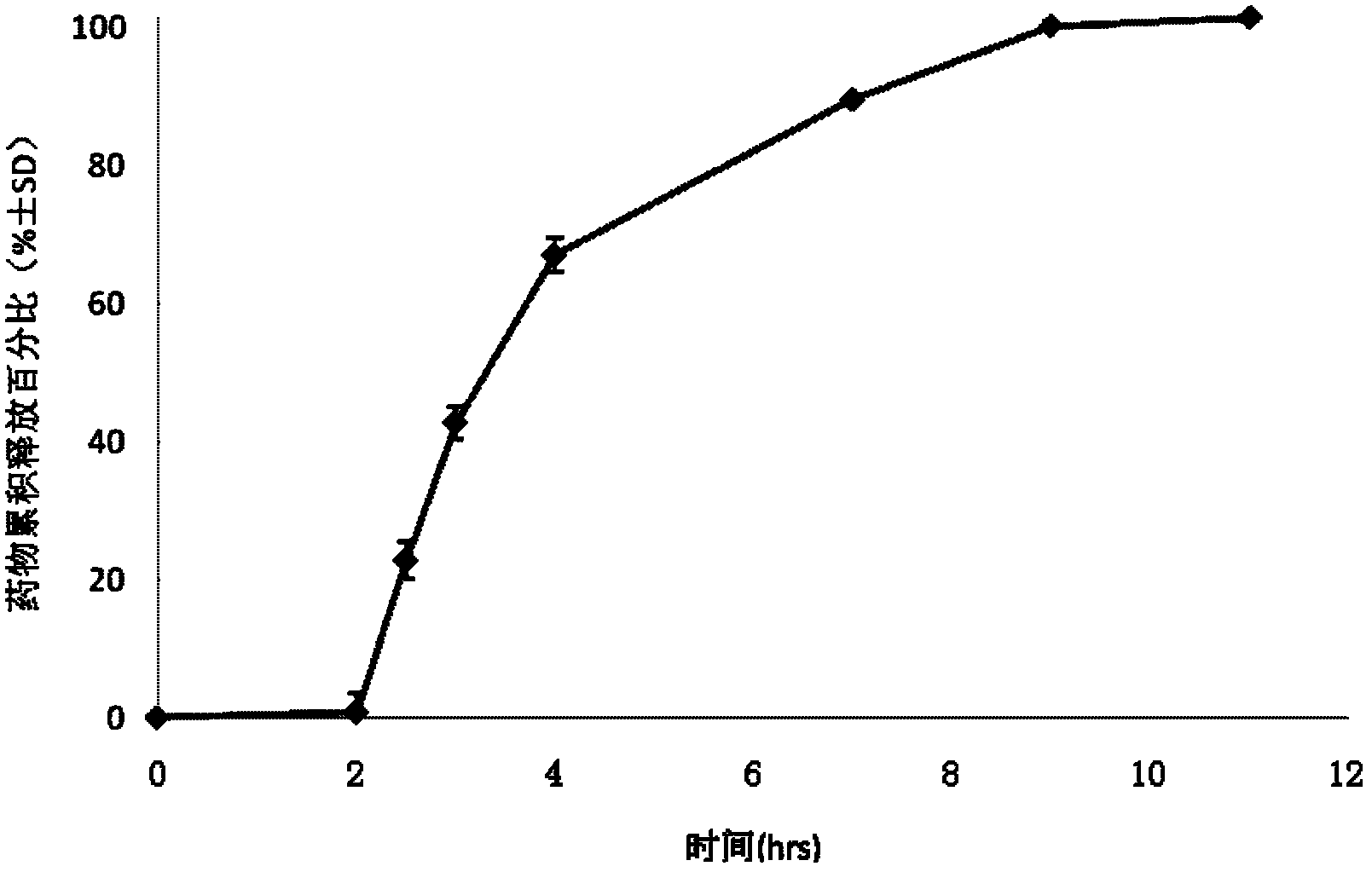
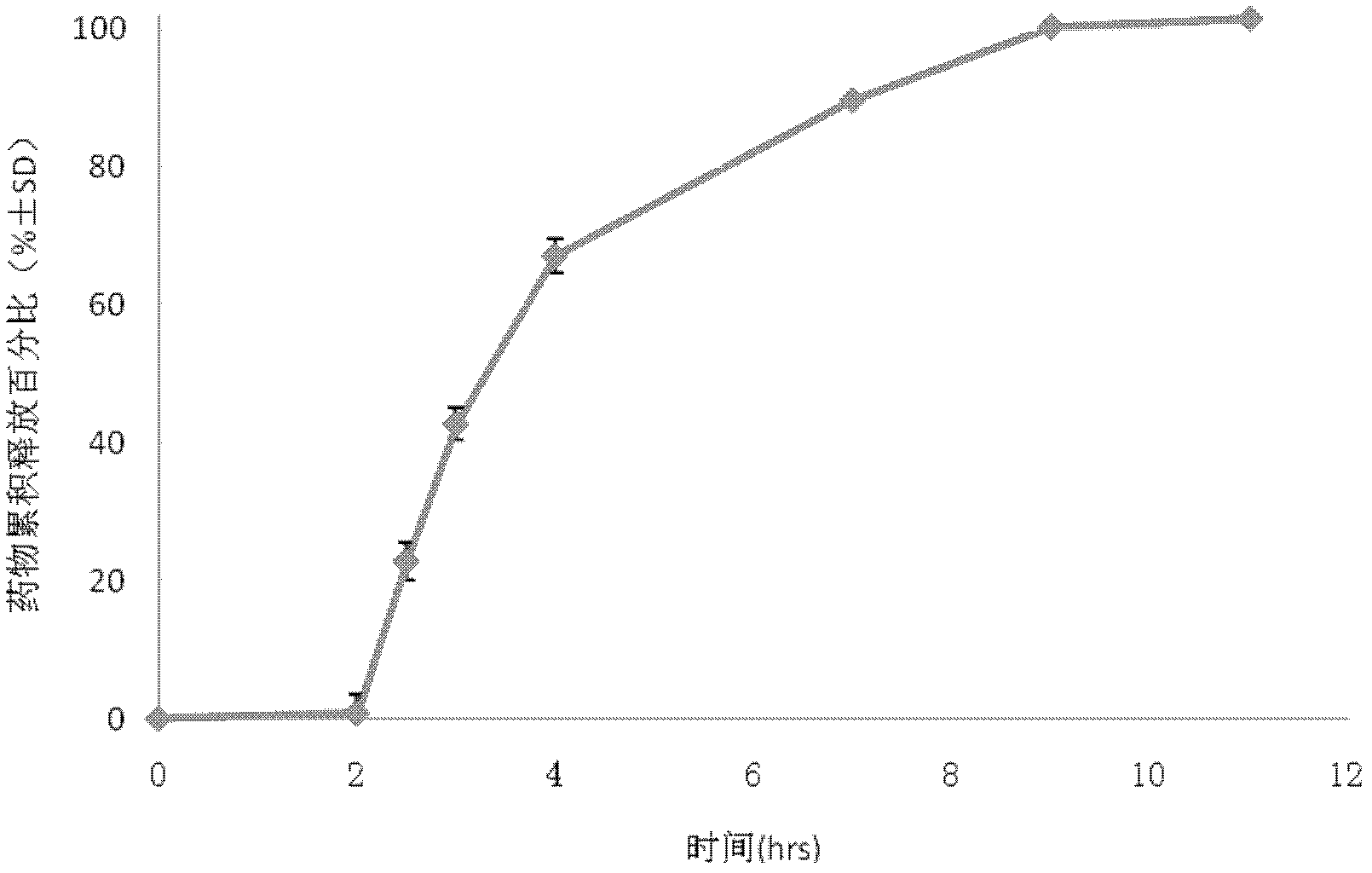
Image
Examples
Embodiment 1
[0039] Prescription with pill core:
[0040] 1 mesalazine 750g
[0041] 2 microcrystalline cellulose 195g
[0042] blank core
[0043] 3 povidone 45g
[0044] 4 water to taste
[0045] Preparation method of ball core: Take the above ingredients 1, 3, and 4 of the drug-containing pill core prescription and stir them evenly to prepare a suspension with a solid content of 10-20% for later use; put the blank core of component 2 into the coating equipment and preheat When the bed temperature is higher than 35°C, pump the suspension of the drug until the end, rinse the container and pipeline with a small amount of water, and dry to obtain a qualified immediate-release drug-containing pellet core.
[0046] Coating prescription:
[0047] 1 povidone 20.0g
[0049] 3 water 270g
[0050] 4 Ethyl cellulose 20.0g
[0052] 6 water 175g
[0053] 7 Methacrylic acid 215.0g
[0054] and methacrylic
[0055] Acid methy...
Embodiment 2
[0061] Prescription with pill core:
[0062] 1 mesalazine 750g
[0063] 2 microcrystalline cellulose 195g
[0064] 3 povidone 45g
[0065] 4 magnesium stearate 10g
[0066] 5 water as appropriate
[0067] Preparation method of pellet core: Take the above components and mix thoroughly, use different types of mixing equipment or high-shear wet granulator, and then add appropriate amount of water to prepare soft material. The obtained soft material is further extruded and granulated, and then spheronized with a spheronizer to obtain a wet pellet core of a suitable size. The pellet core is further dried and sieved to obtain a qualified drug-containing immediate-release pellet core.
[0068] Coating prescription:
[0069] 1 povidone 25.0g
[0071] 3 water 270g
[0072] 4 Ethylcellulose 16.9g
[0073] 5 Talc powder 4.1g
[0074] 6 water 185.0g
[0075] 7 Methacrylic acid 150g
[0076] and acrylic acid
[0077] Ester 1:1 Copolymer
[00...
Embodiment 3
[0085] Prescription with pill core:
[0086] 1 mesalazine 950g
[0087] 2 microcrystalline cellulose 40g
[0088] 3 hypromellose 5g
[0089] white
[0090] 4 magnesium stearate 5g
[0091] 5 water as appropriate
[0092] Preparation method of pellet core: Take the above components and mix thoroughly, use different types of mixing equipment or high-shear wet granulator, and then add appropriate amount of water to prepare soft material. The obtained soft material is further extruded and granulated, and then spheronized with a spheronizer to obtain a wet pellet core of a suitable size. The pellet core is further dried and sieved to obtain a qualified drug-containing immediate-release pellet core.
[0093] Coating prescription:
[0094] 1 povidone 15.0g
[0095] 2 Talc powder 7.5g
[0096] 3 water 210g
[0097] 4 Ethylcellulose 15.9g
[0098] 5 Talc powder 2.1g
[0099] 6 water 165g
[0100] 7 Methacrylic acid 250g
[0101] and methacrylic
[0102] Acid meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com