Colchicines gastric floating sustained-release tablet and method for preparing same
A technology of colchicine and gastric floating, which is applied in the direction of medical formula, medical preparations containing active ingredients, etc., and can solve the problem of high toxicity of colchicine, small gout therapeutic index, and bioavailability Low-level problems, to achieve the effect of improving bioavailability, reducing the number of medications, and stabilizing blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
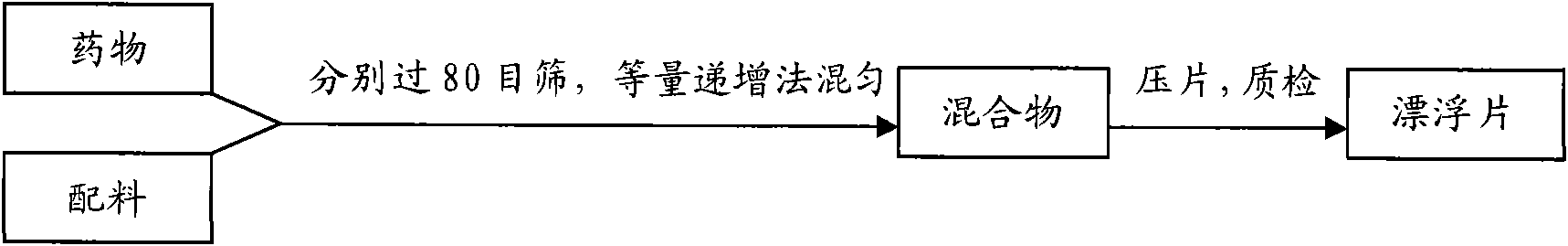
[0074] The preparation process of colchicine gastric floating sustained-release tablets is detailed in figure 1 shown.
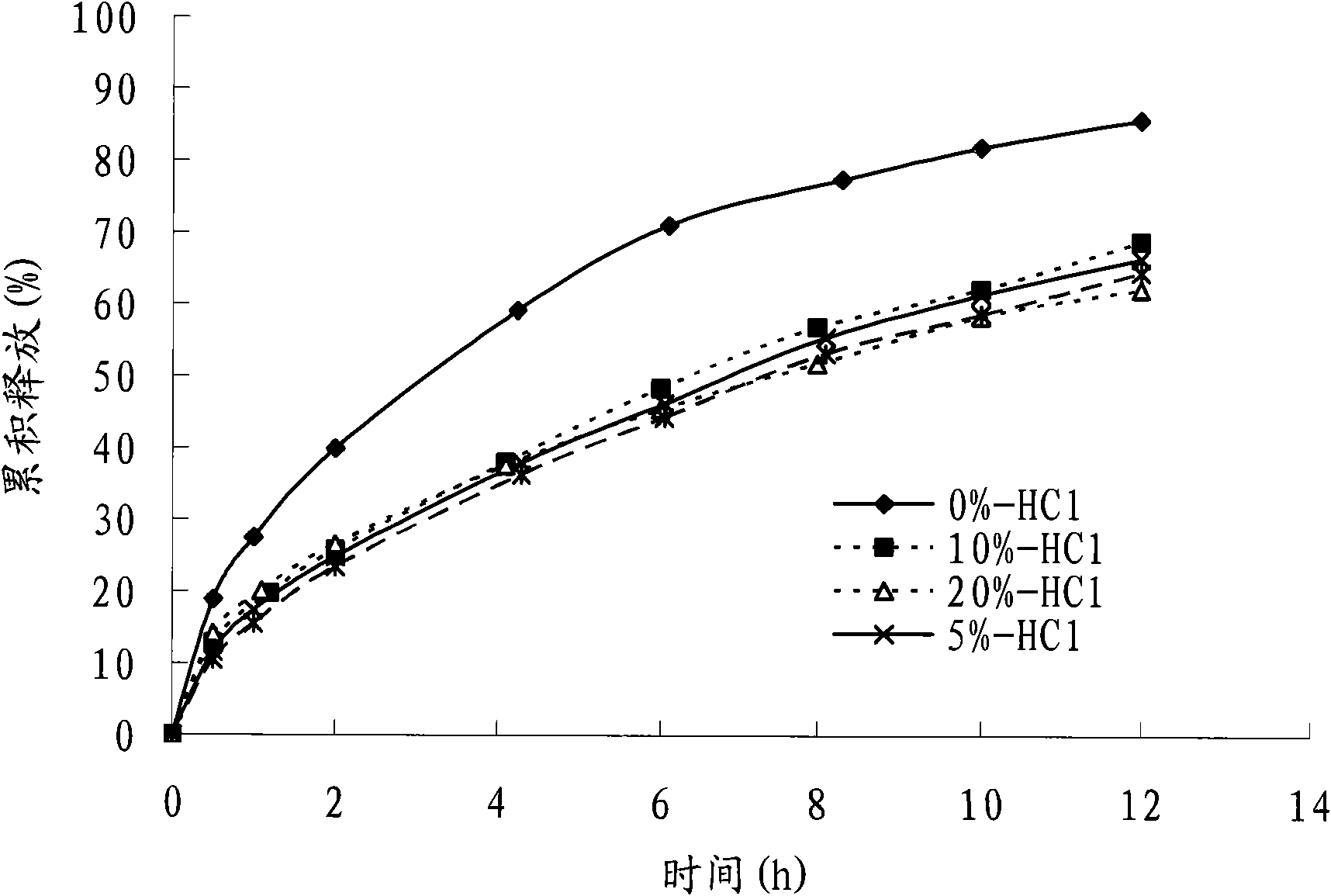
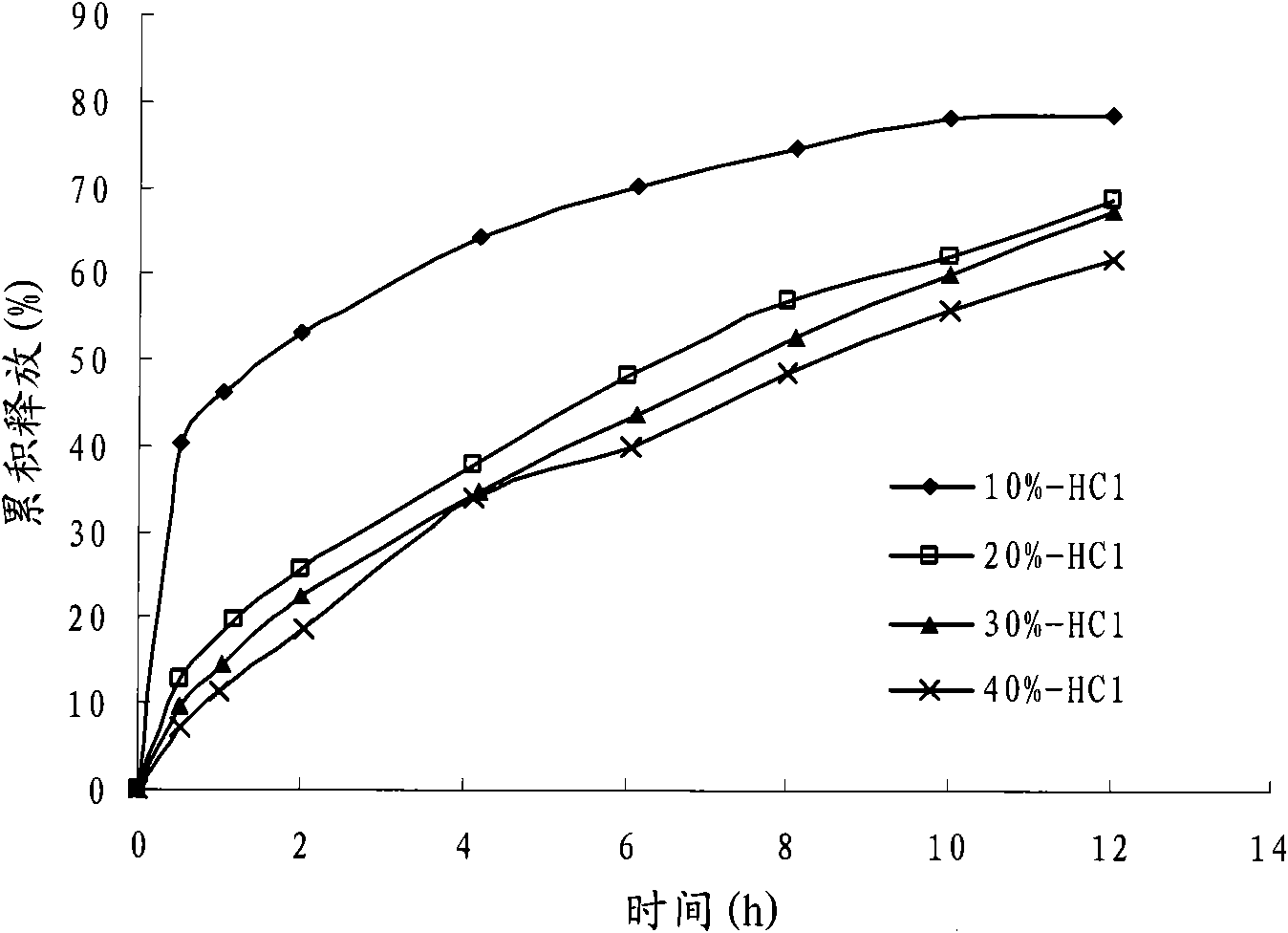
[0075] With reference to relevant bibliographical reports and preliminary experimental results, the present invention adopts similarity factor method (f 2 ) as an evaluation index to investigate the effects of prescription components and preparation process on the release behavior of colchicine matrix controlled-release tablets.
[0076] The basic prescriptions used in the experiment are as follows:
[0077] Colchicine
example 1
[0079] During the experiment, unless otherwise specified, the fixed process parameters were as follows: the raw and auxiliary materials were mixed evenly by the method of equal increment, and the powder was directly compressed into tablets. The die of the tablet press is shallow concave (Φ=7mm); the hardness of the tablet core is 35-45N, and the tablet weight is 150mg±2%.
[0080] In vitro drug release conditions: small cup method, with 200mL of 0.1mol L -1 HCl is the drug release medium, the paddle speed is 100r min -1 , medium temperature 37 ℃.
[0081] 2.1 Prescription single factor investigation
[0082] 2.1.1 The effect of the amount of effervescent agent on drug release behavior
[0083] Fix the dosage of other components in the prescription and the dosage ratio of effervescent agent sodium bicarbonate and citric acid, adjust the ratio of effervescent agent in the prescription, and replace it with the same amount of microcrystalline cellulose, and investigate the eff...
Embodiment 1
[0145] Preparation:
[0146] a. Mix colchicine with polyoxyethylene WSR303, sodium bicarbonate, Eudragit L100-55, microcrystalline cellulose, citric acid, red iron oxide, and magnesium stearate in equal increments;
[0147] b. Carry out direct powder compression, the die of the tablet machine is shallow concave, Φ=7mm; tablet core hardness 35-45N, tablet weight 150mg±2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com