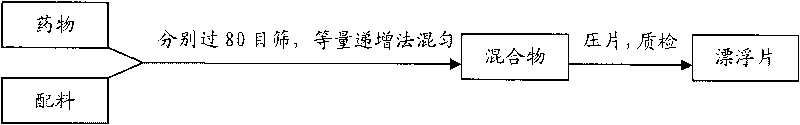
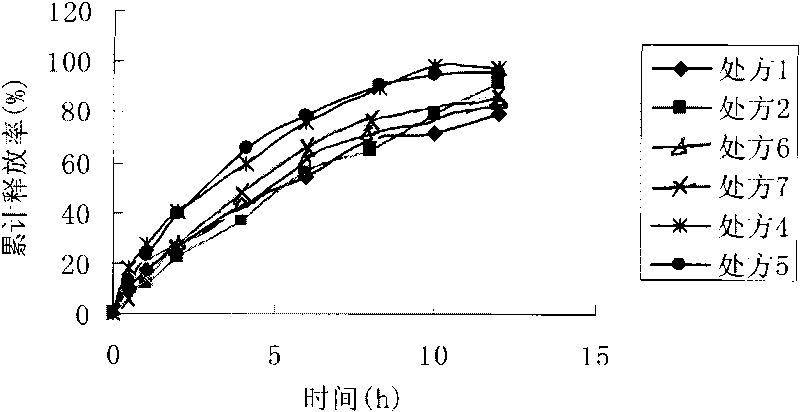
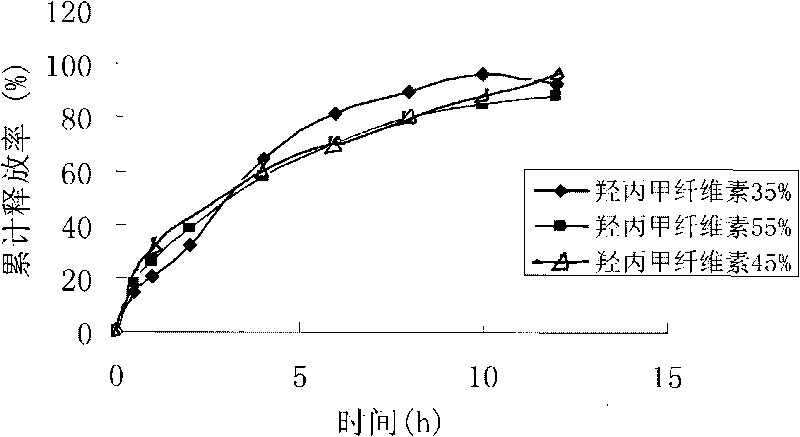
Huperzine-A intra-gastric floating tablet
A technology of huperzine A and gastric floating tablets, which is applied in the fields of pill delivery, organic active ingredients, nervous system diseases, etc., can solve problems such as difficult control of quality stability, insufficient drug release, and inconvenient medication for patients, so as to improve biological Utilization, constant and complete drug release, and the effect of reducing the number of doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] prescription
[0109] Huperzine A 20g
[0110] Hypromellose K15M 300g
[0111] Crospovidone 92g
[0112] Citric acid 25.2g
[0113] Sodium bicarbonate 34.8g
[0114] Microcrystalline Cellulose 260g
[0115] Lactose 240g
[0116] Magnesium Stearate Appropriate amount
[0117] Pigment 5g
[0118] Made into 1000g
[0119] making process:
[0120] The raw material drug huperzine A and the auxiliary materials that have passed through the 80-mesh sieve were weighed respectively according to the prescription amount, and the raw and auxiliary materials were mixed evenly by the method of equal increment, and the powder was directly compressed into tablets. The die of the tablet press is shallow concave (Φ=7mm); the hardness of the tablet core is 35-45N, and the tablet weight is 150mg±2%.
Embodiment 2
[0122] Prescription Huperzine A 50g
[0123] Hypromellose K15M 300g
[0124] Crospovidone 80g
[0125] Citric acid 33.6g
[0126] Sodium bicarbonate 46.4g
[0127] Microcrystalline Cellulose 232g
[0128] Lactose 230g
[0129] Magnesium Stearate Appropriate amount
[0130] Pigment 5g
[0131]
[0132] Made into 1000g
[0133] Preparation process: with embodiment 1.
Embodiment 3
[0135] prescription
[0136] Huperzine A 0.5g
[0137] Hypromellose K100M 400g
[0138] Crospovidone 250g
[0139] Sodium bicarbonate 10g
[0140] Microcrystalline Cellulose 160g
[0141] Lactose 151.5g
[0142] Magnesium Stearate Appropriate amount
[0143] Pigment 5g
[0144]
[0145] Made into 1000g
[0146] Preparation process: with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com