Pharmaceutical composition containing gliclazide
A Glichetide and composition technology, applied in the field of sustained-release pharmaceutical compositions, can solve problems such as poor water solubility and incomplete release of the main drug, and achieve the effect of increasing water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
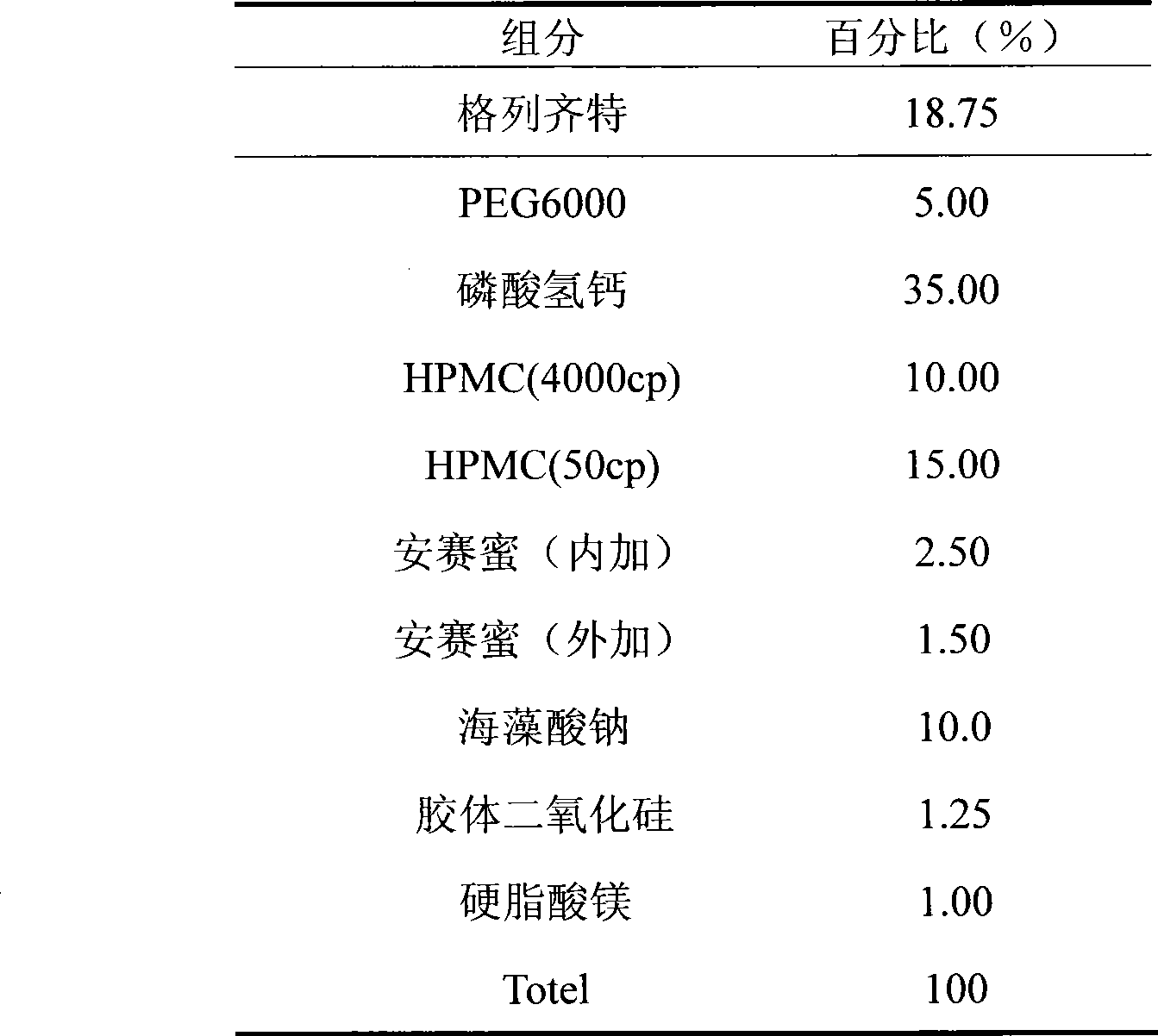
[0014] This embodiment is made into a solid dispersion first and then into a sustained-release tablet
[0015]
[0016] Preparation process: weigh gliclazide and PEG6000 according to the prescription amount, use 95% ethanol solution as a binder, add hypromellose, and wet granulate with 60 mesh. Dried at 50°C, crushed and stirred with calcium hydrogen phosphate, sodium alginate, acesulfame potassium and colloidal silicon dioxide to dissolve, 30 mesh wet granulation, and dried at 60°C. Whole grains with 26 meshes, measure the water content, add magnesium stearate and acesulfame potassium, mix, and compress into tablets at 60-80N.
Embodiment 2
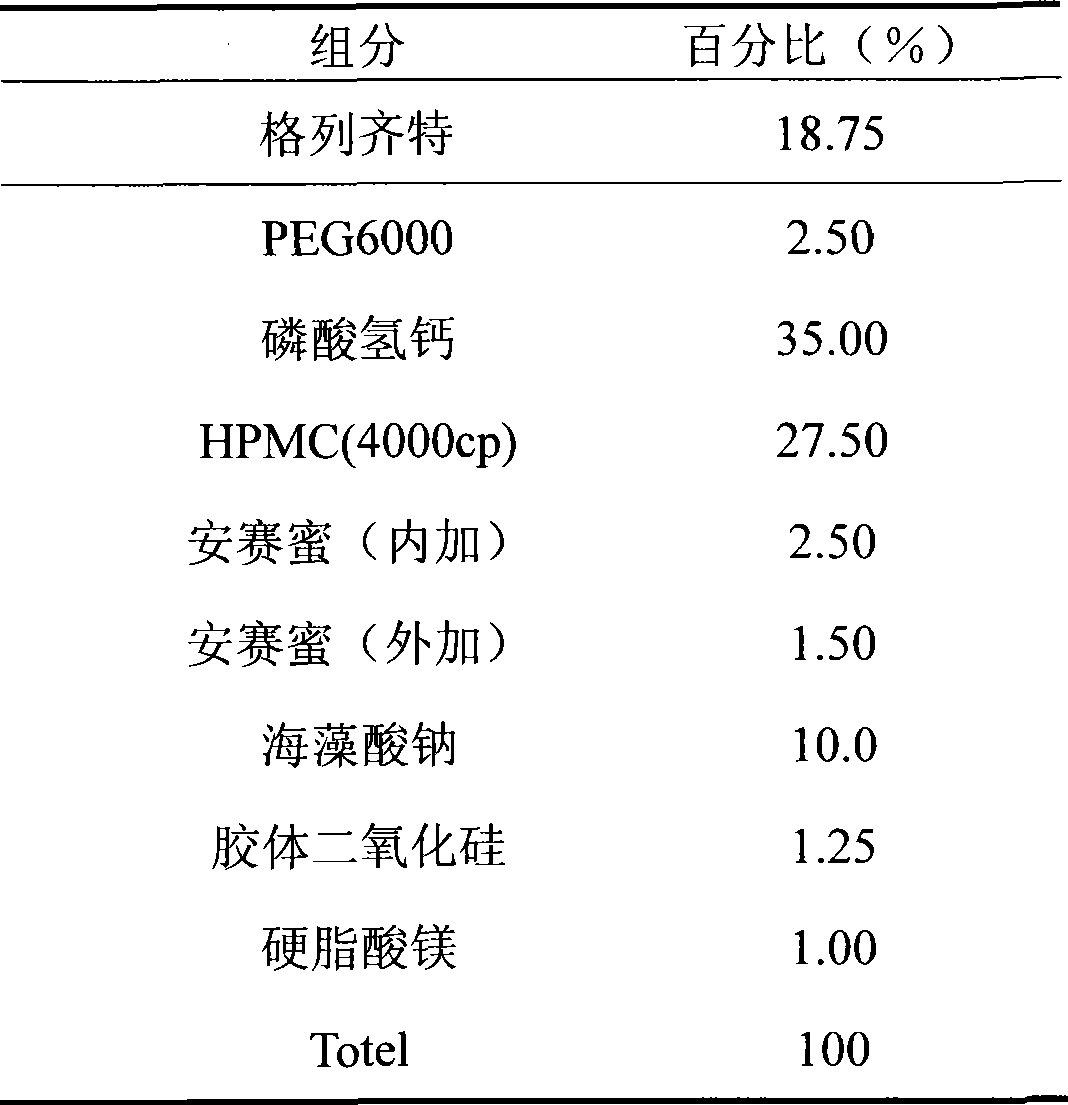
[0018] This embodiment is made into a solid dispersion first and then into a sustained-release tablet
[0019]
[0020] Preparation process: weigh gliclazide and PEG6000 according to the prescription amount, use 95% ethanol solution as a binder, add hypromellose, and wet granulate with 60 mesh. Dried at 50°C, crushed and stirred with calcium hydrogen phosphate, sodium alginate, acesulfame potassium and colloidal silicon dioxide to dissolve, 30 mesh wet granulation, and dried at 60°C. Whole grains with 26 meshes, measure the water content, add magnesium stearate and acesulfame potassium, mix, and compress into tablets at 60-80N.
Embodiment 3
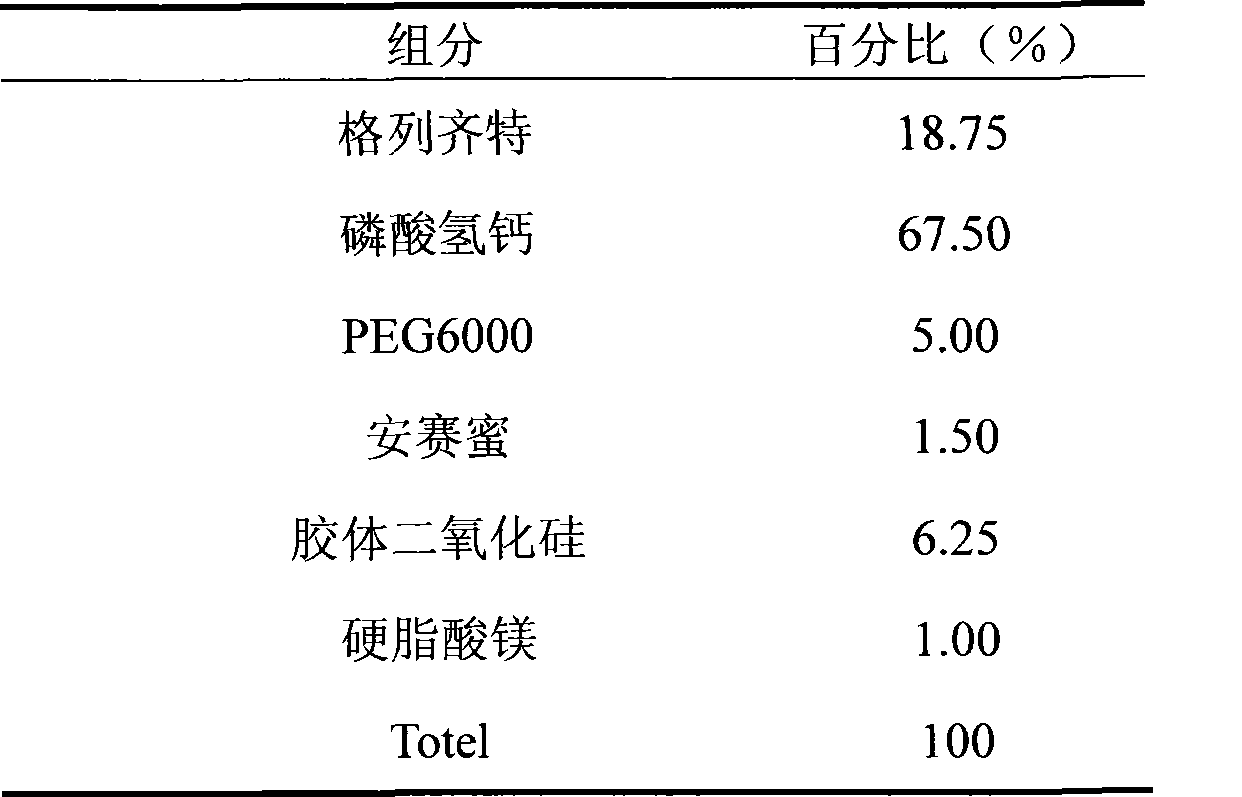
[0022] This embodiment mode directly makes sustained-release tablet as
[0023]
[0024] Preparation process: Weigh gliclazide, calcium hydrogen phosphate and PEG6000 according to the prescription, use 95% ethanol solution as a binder, add acesulfame potassium and colloidal silicon dioxide and stir to dissolve them, 30 mesh wet granulation, Dry at 60°C. The granules are sized at 26 mesh, the water content is measured, magnesium stearate is added and mixed, and then pressed into tablets at 60-80N.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com