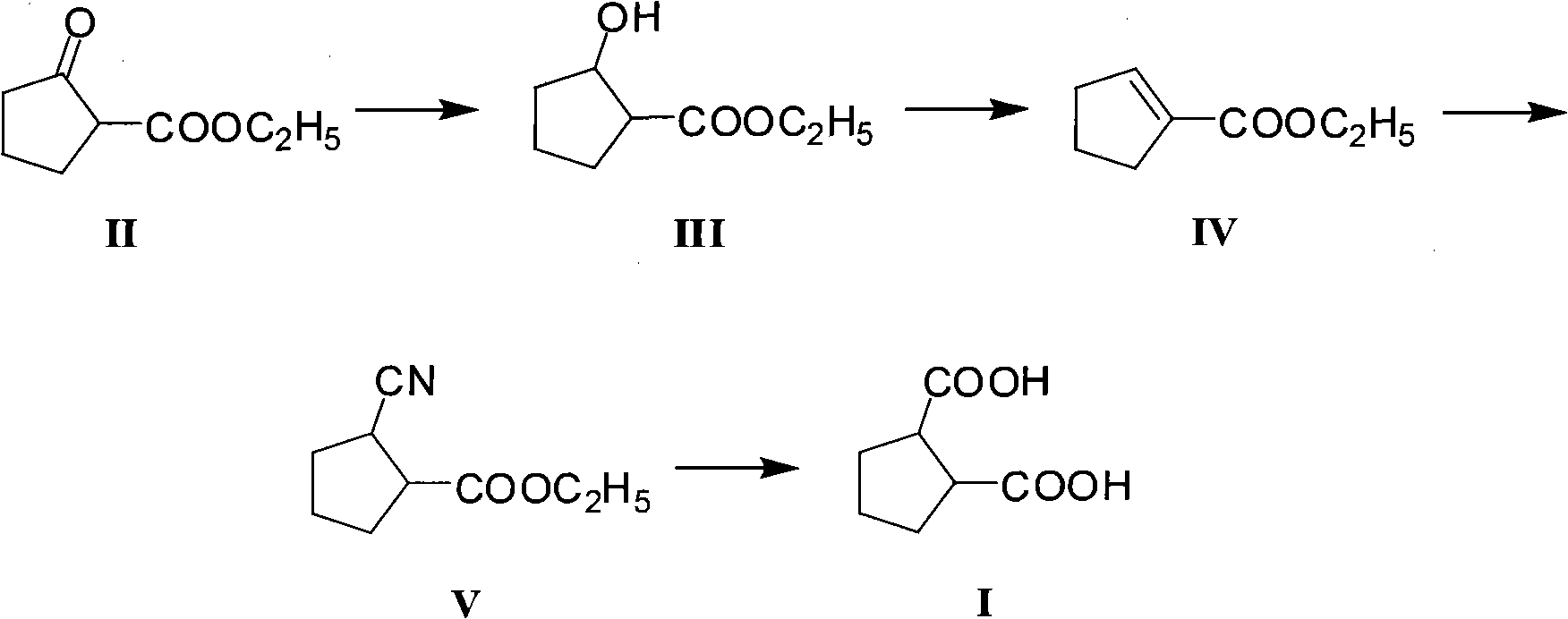
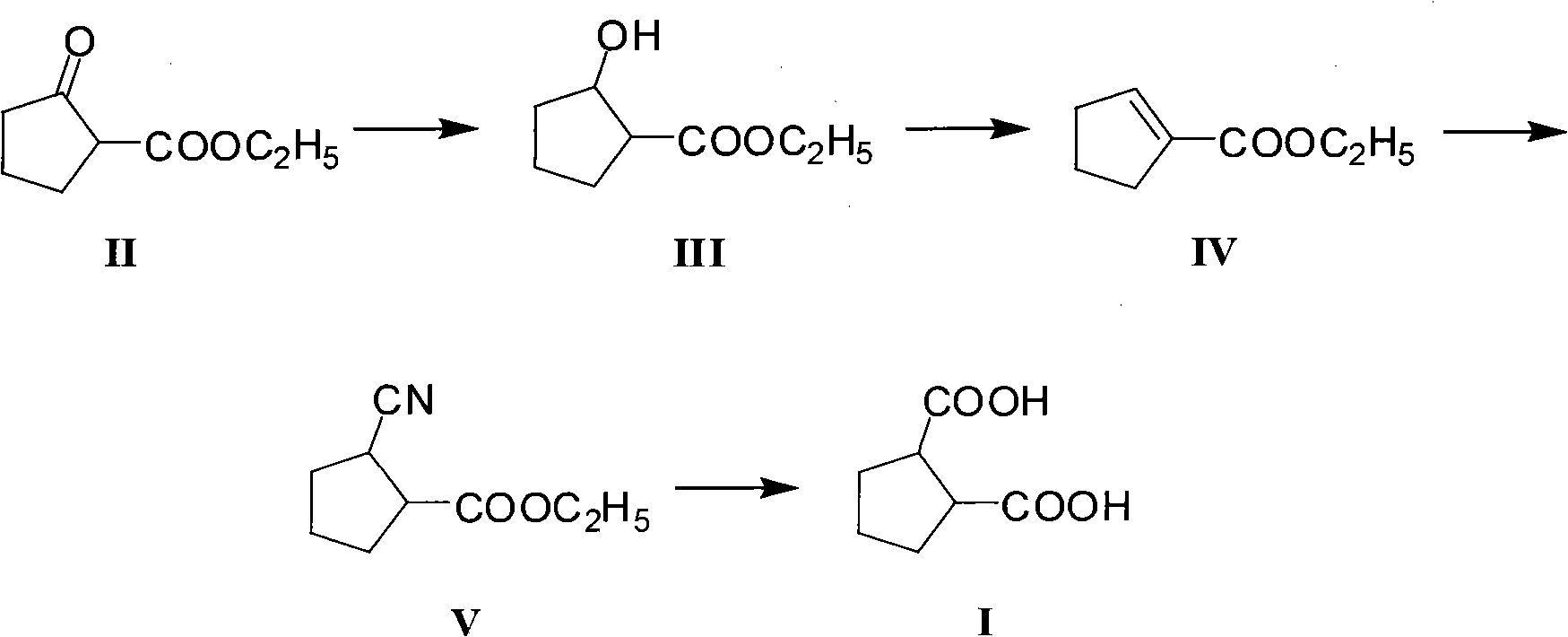
Method for preparing intermediate 1,2-dicarboxylicacid of antidiabetic medicine gliclazide
A technology of cyclopentanedicarboxylic acid and ethyl cyclopentamate, which is applied in the field of hypoglycemic drug gliclazide intermediate 1, can solve the problems of difficult availability, harsh reaction conditions, high temperature and long time reaction, etc., and achieves the reaction conditions Moderate, sufficient market supply, cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A. Preparation of ethyl 2-hydroxycyclopentylcarboxylate (III)
[0033] In a reactor equipped with a mechanical stirring device, a reflux condenser, and a mercury thermometer, add ethyl 2-oxocyclopentanoate (15.6g, 0.1mol) absolute ethanol (155ml), stir and cool with an ice bath to 0°C, and then add sodium borohydride (2.28g, 0.06mol) in batches within 1 hour. After the addition, remove the ice bath, naturally rise to room temperature, and continue stirring for 6 hours. After the reaction is complete, add 5% dilute hydrochloric acid (124g), the mixed solution is extracted with dichloromethane again, and the dichloromethane layer is dried with anhydrous sodium sulfate after the liquid separation, and after the desiccant is removed by filtration, the solvent is distilled off to obtain the crude product of ethyl 2-hydroxycyclopentylcarboxylate (III), About 15.3g, the yield is about 96.8%, the crude product can be directly used in the next reaction without further purificati...
Embodiment 2
[0041] Other steps are identical with embodiment 1, just the preparation method of the 2-hydroxycyclopentyl ethyl carboxylate (III) of A step is as follows:
[0042] In a reactor equipped with a mechanical stirring device, a reflux condenser, and a mercury thermometer, add ethyl 2-oxocyclopentanoate (15.6g, 0.1mol) absolute ethanol (100ml), stir and cool with an ice bath to 0°C, then add sodium borohydride (1.9 g, 0.05 mol) in batches within 1 hour, remove the ice bath after the addition, naturally rise to room temperature, continue stirring for 4 hours, and then add 5% dilute hydrochloric acid (78g), the mixed solution is extracted with dichloromethane again, and the dichloromethane layer is dried with anhydrous sodium sulfate after the liquid separation, and after the desiccant is removed by filtration, the solvent is distilled off to obtain the crude product of ethyl 2-hydroxycyclopentylcarboxylate (III), About 14.1 g, the yield is about 89.2%, the crude product can be dire...
Embodiment 3
[0044] Other steps are identical with embodiment 1, just the preparation method of the 2-hydroxycyclopentyl ethyl carboxylate (III) of A step is as follows:
[0045] In a reactor equipped with a mechanical stirring device, a reflux condenser, and a mercury thermometer, add ethyl 2-oxocyclopentanoate (15.6g, 0.1mol) anhydrous methanol (155ml), stir and cool with an ice bath to 0°C, and then add potassium borohydride (3.24g, 0.06mol) in batches within 1 hour, remove the ice bath after the addition, naturally rise to room temperature, continue to stir and react for 6 hours, and then add 5% dilute hydrochloric acid (124g), the mixed solution is extracted with dichloromethane again, and the dichloromethane layer is dried with anhydrous sodium sulfate after the liquid separation, and after the desiccant is removed by filtration, the solvent is distilled off to obtain the crude product of ethyl 2-hydroxycyclopentylcarboxylate (III), About 15 g, the yield is about 94.9%, the crude pro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com