Method for preparing intermediate 1,2-dicarboxylicacid of antidiabetic medicine gliclazide
A technology of cyclopentanedicarboxylic acid and ethyl cyclopentamate, which is applied in the field of hypoglycemic drug gliclazide intermediate 1, can solve the problems of harsh reaction conditions, not easy to obtain, high price and the like, and achieves sufficient market supply, Mild reaction conditions and low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
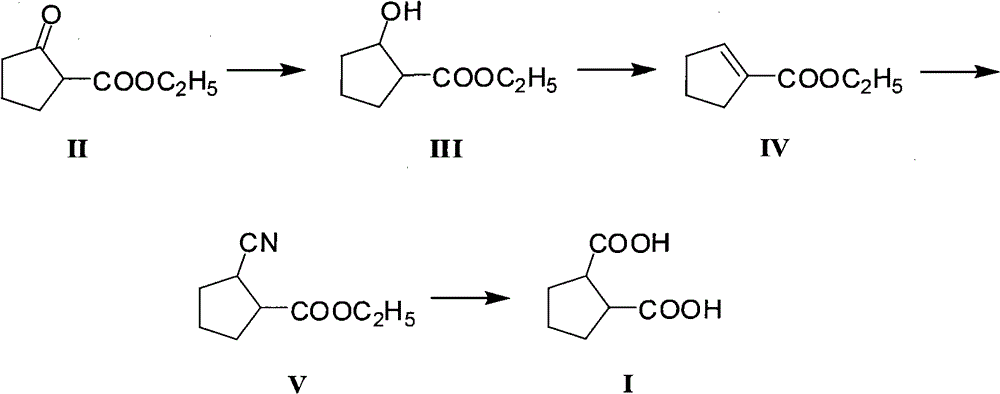
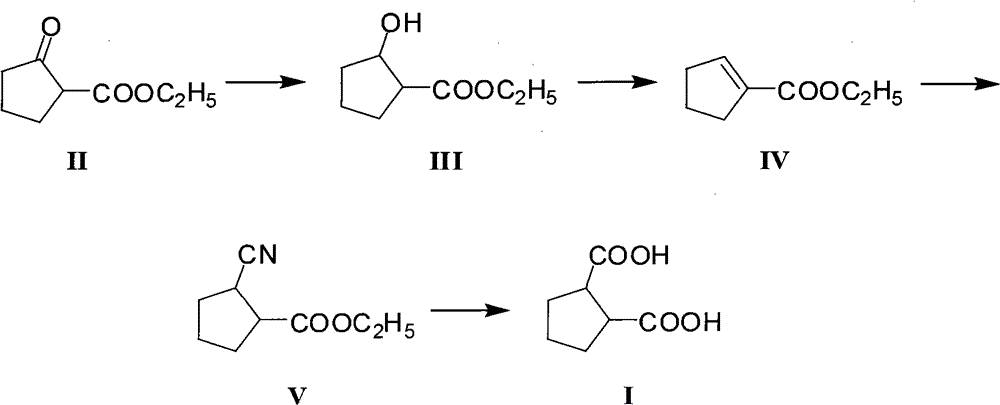
[0032] A. Preparation of ethyl 2-hydroxycyclopentylcarboxylate (III)
[0033] In a reactor equipped with a mechanical stirring device, a reflux condenser, and a mercury thermometer, add ethyl 2-oxocyclopentacarboxylate (15.6g, 0.1mol) anhydrous ethanol (155ml), stir and stir well, then cool with an ice bath To 0℃, then add sodium borohydride (2.28g, 0.06mol) in batches within 1 hour, remove the ice bath after the addition is complete, and naturally rise to room temperature, continue to stir and react for 6 hours, and then add 5% dilute hydrochloric acid (124g), the mixed solution was extracted with dichloromethane. After liquid separation, the dichloromethane layer was dried with anhydrous sodium sulfate, filtered to remove the desiccant, and the solvent was distilled off to obtain crude ethyl 2-hydroxycyclopentylcarboxylate (III). About 15.3g, the yield is about 96.8%, the crude product can be used directly in the next step without further purification.
[0034] B. Preparation of...
Embodiment 2
[0041] The other steps are the same as in Example 1, except that the preparation method of ethyl 2-hydroxycyclopentylcarboxylate (III) in step A is as follows:
[0042] In a reactor equipped with a mechanical stirring device, a reflux condenser, and a mercury thermometer, add ethyl 2-oxocyclopentacarboxylate (15.6g, 0.1mol) absolute ethanol (100ml), stir and stir well, then cool with an ice bath To 0°C, then add sodium borohydride (1.9g, 0.05mol) in batches within 1 hour, remove the ice bath after the addition is complete, naturally warm to room temperature, continue to stir and react for 4 hours, and then add 5% dilute hydrochloric acid (78g), the mixed solution was extracted with dichloromethane. After separation, the dichloromethane layer was dried with anhydrous sodium sulfate, filtered to remove the desiccant, and the solvent was distilled off to obtain a crude product of ethyl 2-hydroxycyclopentylcarboxylate (III). About 14.1g, the yield is about 89.2%, the crude product ca...
Embodiment 3
[0044] The other steps are the same as in Example 1, except that the preparation method of ethyl 2-hydroxycyclopentylcarboxylate (III) in step A is as follows:
[0045] In a reactor equipped with a mechanical stirring device, a reflux condenser, and a mercury thermometer, add ethyl 2-oxocyclopentacarboxylate (15.6g, 0.1mol) anhydrous methanol (155ml), stir and stir well, then cool with an ice bath To 0°C, then add potassium borohydride (3.24g, 0.06mol) in batches within 1 hour, remove the ice bath after the addition is complete, naturally warm to room temperature, continue to stir and react for 6 hours, and then add 5% dilute hydrochloric acid (124g), the mixed solution was extracted with dichloromethane. After liquid separation, the dichloromethane layer was dried with anhydrous sodium sulfate, filtered to remove the desiccant, and the solvent was distilled off to obtain crude ethyl 2-hydroxycyclopentylcarboxylate (III). About 15g, the yield is about 94.9%, the crude product can...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com