Nequinate preparation method
A technology of trimethoprim and benzyloxy, which is applied in the field of preparation of anticoccidial drugs, can solve the problem of limited supply of dimethyl methoxymethylenemalonate, low market share, and restrictions on large-scale production. Mass production and other issues to achieve the effect of improving production safety, sufficient market supply, and cheap prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
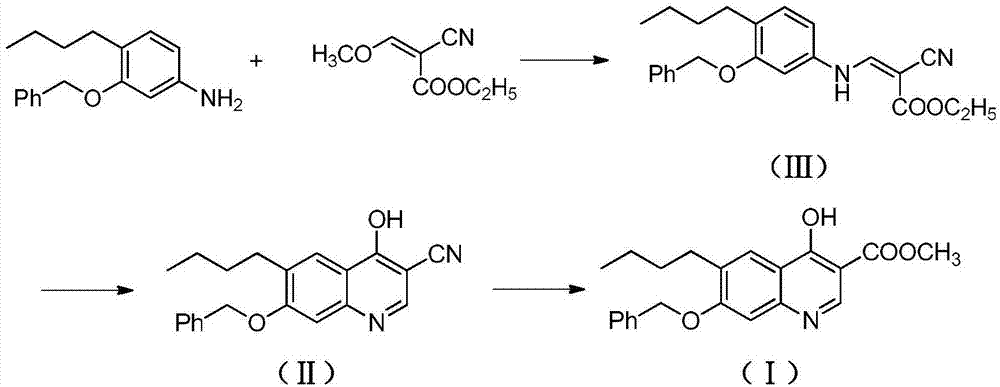
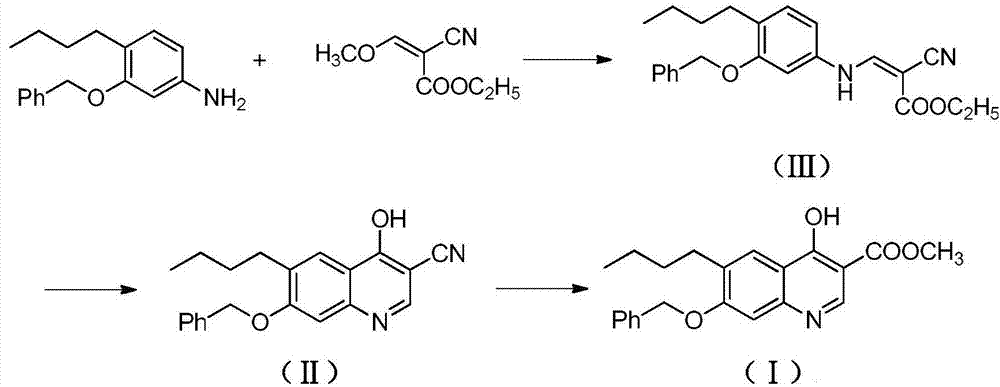
[0022] A. Preparation of ethyl 3-(3-benzyloxy-4-butylanilino)-2-cyanoacrylate (Ⅲ)
[0023] Add 4-butyl-3-benzyloxyaniline (255g, 1.0mol), ethyl 2-cyano-3-methoxyacrylate (186g, 1.2mol) in the reactor, stir and heat up to 30- React at 35°C, continue to stir and react for 8 hours. After the reaction is completed, the temperature is raised to 65°C under normal pressure to distill off the methanol generated by the reaction. The residue contains about 3-(3-benzyloxy-4-butylanilino)-2- Ethyl cyanoacrylate (Ⅲ) was 337.2g, with a yield of about 89.2%, which could be directly used in the next reaction.
[0024] B. Preparation of 7-benzyloxy-6-butyl-4-cyano-3-cyanoquinoline (Ⅱ)
[0025] Add 3-(3-benzyloxy-4-butylanilino)-2-cyanoacrylate ethyl ester (Ⅲ) (378g, 1.0mol) and triethyl phosphate (3000g) into the reactor, stir well at room temperature Afterwards, the temperature was raised to 120° C., and the stirring reaction was continued for 10 hours. After the reaction was completed, it ...
Embodiment 2
[0031] Other steps are the same as in Example 1, except that the preparation method of 3-(3-benzyloxy-4-butylanilino)-2-cyanoacrylate ethyl ester (Ⅲ) of A step is as follows:
[0032] In the reactor, add 4-butyl-3-benzyloxyaniline (255g, 1.0mol), 2-cyano-3-methoxyethyl acrylate (155g, 1.0mol), stir and heat up to 30- React at 35°C and continue to stir for 4 hours. After the reaction is completed, the temperature is raised to 65°C under normal pressure to distill off the methanol generated by the reaction. The residue contains about 3-(3-benzyloxy-4-butylanilino)-2- Ethyl cyanoacrylate (Ⅲ) was 283.6g, with a yield of about 75.0%, which could be directly used in the next reaction.
Embodiment 3
[0034] Other steps are the same as in Example 1, except that the preparation method of 3-(3-benzyloxy-4-butylanilino)-2-cyanoacrylate ethyl ester (Ⅲ) of A step is as follows:
[0035] Add 4-butyl-3-benzyloxyaniline (255g, 1.0mol), ethyl 2-cyano-3-methoxyacrylate (170.5g, 1.1mol) in the reactor, stir and heat up to 30 React at -35°C, continue to stir and react for 5 hours. After the reaction is completed, the temperature is raised to 65°C under normal pressure to distill off the methanol generated by the reaction. The residue contains about 3-(3-benzyloxy-4-butylanilino)-2 - 308.9 g of ethyl cyanoacrylate (Ⅲ), with a yield of about 81.7%, which can be directly used in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com