Gliclazide sustained-release tablets
A technology for gliclazide and sustained-release tablets, which is applied in the field of gliclazide sustained-release tablets and their preparation, can solve the problems of reaching a very high blood drug concentration, low medication compliance of patients, and many daily doses, and the like. The effect of stable drug release rate, good drug stability and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
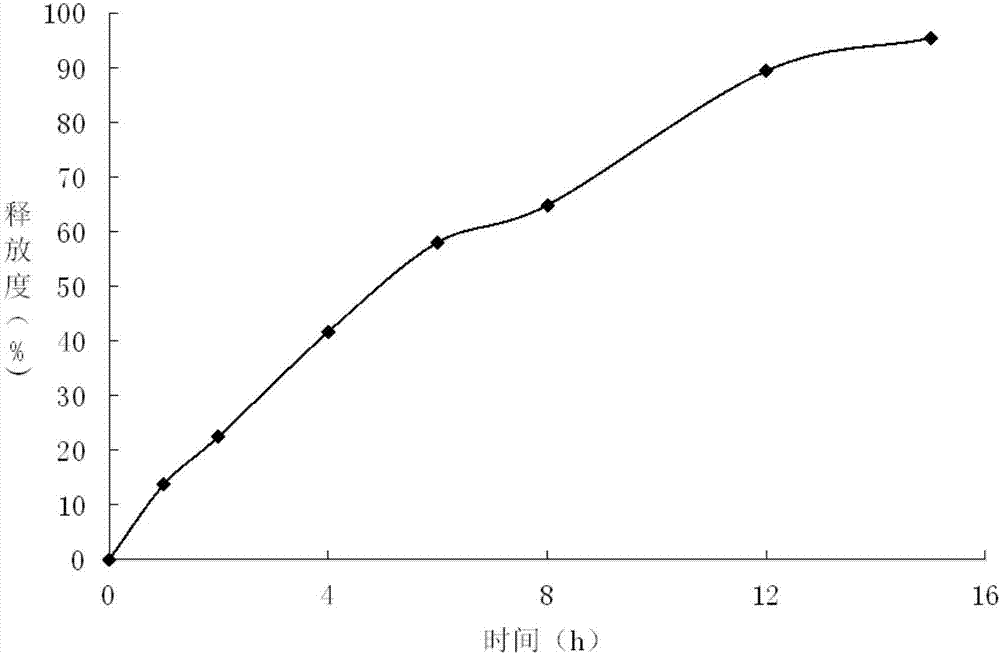
Embodiment 1
[0028] Embodiment 1 Meglumine dosage screening test
[0029] Weigh glyceryl behenate, divide it into 6 equal portions, 90g each, heat to 65-70°C to melt respectively, add meglumine 1, 2, 4, 8, 12, 16g, under the condition of keeping warm at 65-70°C, add 30g of gliclazide respectively, stir for 60min with an electric stirrer, and observe whether the gliclazide melts.
[0030] Table 1: Meglumine Dosage Screening
[0031] Meglumine Dosage
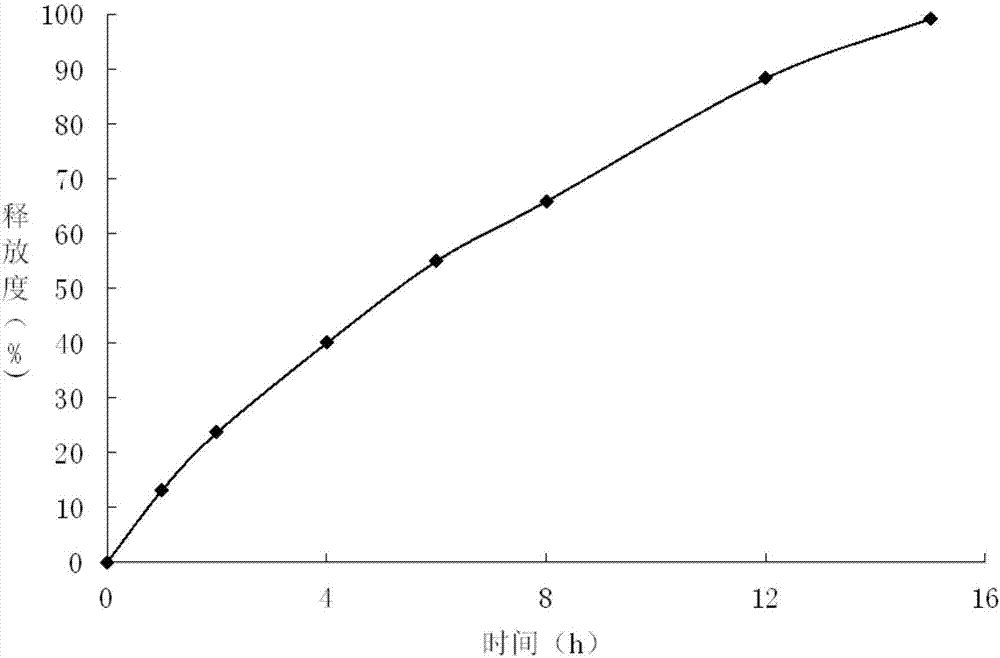
Embodiment 2
[0032] Preparation of Embodiment 2 Gliclazide Sustained-release Tablets
[0033]
[0034] Preparation Process:
[0035] ①Take glyceryl behenate, heat to 65-70°C to melt, keep warm and add the prescribed amount of meglumine while stirring;
[0036] ②Continue to keep warm at 65-70°C, add gliclazide at the same time, use an electric stirrer to stir for more than 30 minutes until it melts, quickly put it into ice-water mixture to solidify, and put it in a desiccator to dry after it is completely solidified, and the drying temperature is lower than 60 ℃, drying time is 2h, grind, and pass through 80 mesh sieve to obtain drug-loaded granules;
[0037] ③Mix the drug-loaded granules with the prescribed amount of mannitol (passed through a 100-mesh sieve) and magnesium stearate evenly, and press into tablets, Φ9mm punched tablets, and control the hardness within the range of 40-60N.
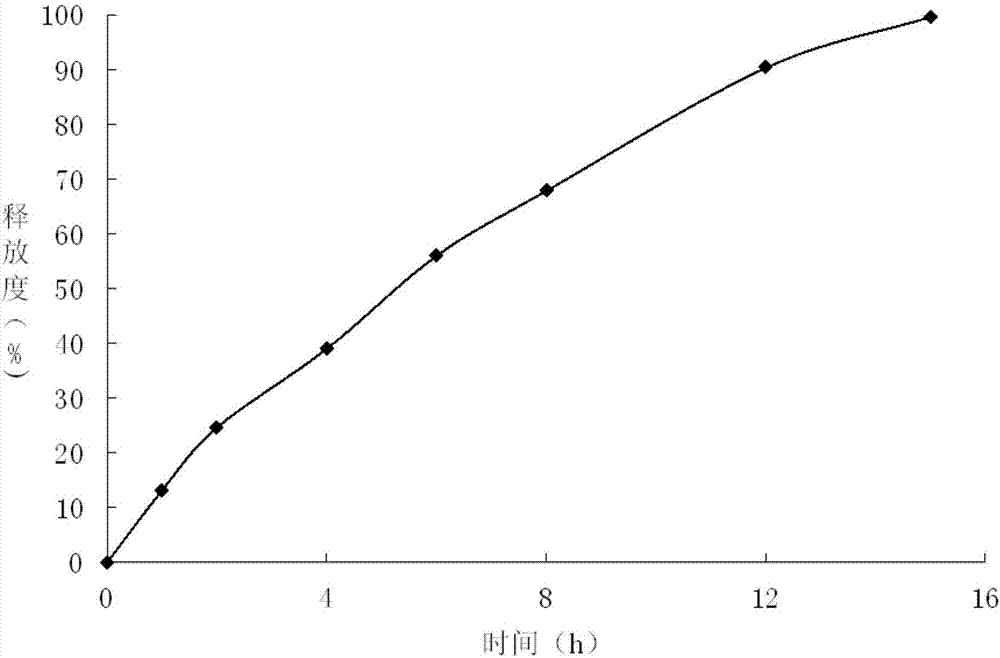
Embodiment 3
[0038] Preparation of Example 3 Gliclazide Sustained-release Tablets
[0039]
[0040]
[0041] Preparation Process:
[0042]①Take glyceryl behenate, heat to 65-70°C to melt, keep warm and add the prescribed amount of meglumine while stirring;
[0043] ②Continue to keep warm at 65-70°C, add gliclazide at the same time, use an electric stirrer to stir for more than 30 minutes until it melts, quickly put it into ice-water mixture to solidify, and put it in a desiccator to dry after it is completely solidified, and the drying temperature is lower than 60 ℃, drying time is 2h, grind, and pass through 80 mesh sieve to obtain drug-loaded granules;
[0044] ③Mix the drug-loaded granules with the prescribed amount of mannitol (passed through a 100-mesh sieve) and magnesium stearate evenly, and press into tablets, Φ9mm punched tablets, and control the hardness within the range of 40-60N.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com