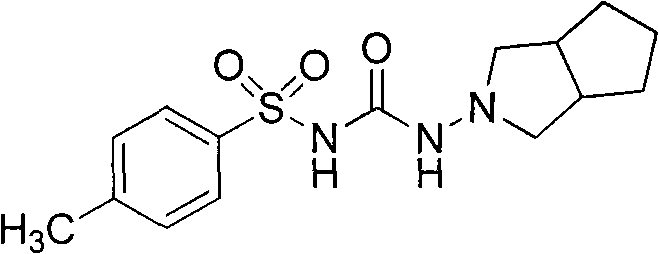
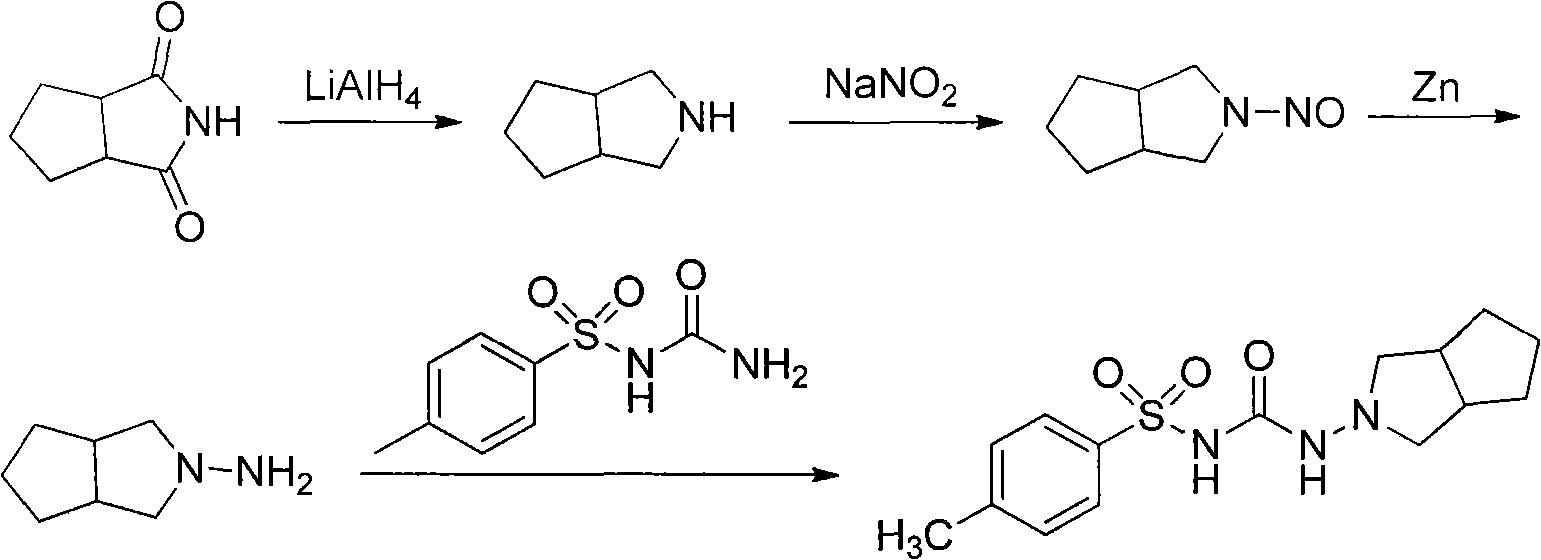
Preparation method of gliclazide
A technology of cyclopentane ortho, hydrazine hydrate, applied in the field of medicine, can solve the problems of inconvenient setting, high price, high reaction cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: N-amino-1,2-cyclopentane phthalimide
[0016] Add 1,2-cyclopentane phthalic anhydride (50 g, 357 mmol) and 250 mL of ethanol into a 1 L four-ring bottle equipped with a mechanical stirrer, a condenser, and a dropping funnel, and heat to reflux. Slowly add 80% hydrazine hydrate (24.4 mL, 375 mmol) dropwise, and continue to reflux after the dropwise addition, and follow the gas phase to complete the reaction. The reaction takes about 8 hours. Heating was stopped, and it was cooled down to room temperature naturally, and the solvent was removed by rotary evaporation under reduced pressure to obtain a viscous liquid, and the solid was washed out after cooling. Crush the solid, beat with 50mL of cold water for half an hour under ice-water bath cooling, filter with suction, and dry the solid in a vacuum oven at 30°C for 12 hours to obtain 46g of the product, with a yield of 83.6%, a melting point of 11.3-114.6°C, and a gas-phase purity of 98.2%. .
Embodiment 2
[0017] Example 2: Hexahydro-2-cyclopentopyrrolylamine hydrochloride
[0018] Carefully dilute 75.0mL of concentrated sulfuric acid into 200mL of tetrahydrofuran, turn black, and let it cool to room temperature.
[0019] Add N-amino-1,2-cyclopentane phthalimide (212.7g, 1.38mol) and 1.5L tetrahydrofuran into a 3L three-necked flask, stir at room temperature until the solid is completely dissolved, and add hydroboration in batches Sodium (130 g, 3.45 mol). The system was cooled under an ice-water bath. When the temperature of the system drops to 5-10°C, dilute concentrated sulfuric acid in tetrahydrofuran is added dropwise. After the dropwise addition is completed, heat to reflux and follow the gas phase to complete the reaction, which takes about 14-16 hours.
[0020] After the reaction was finished, it was cooled down to room temperature naturally, and methanol was added dropwise to quench the reaction, and stirred for 30 min. Most of the solvent (about 1 L) was evaporated ...
Embodiment 3
[0023] Embodiment 3: Preparation of Gliclazide
[0024] Add hexahydro-2-cyclopentapyrrolylamine hydrochloride (20g, 123mmol), p-toluenesulfonylurea (29g, 135mmol), 100mL toluene in the 250mL three-strand bottle equipped with mechanical stirring and condenser, and heat Reflux, TLC tracking reaction complete ester, about 2 ~ 3h. After the reaction, the toluene was distilled off under reduced pressure, 100 mL of water was added, and the crystallization was stirred at room temperature for 12 h. Suction filtration, wash the solid with a small amount of cold water, recrystallize from ethyl acetate, and dry in a vacuum oven at 80°C for 12 hours to obtain 34.1 g of the product with a yield of 85.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com