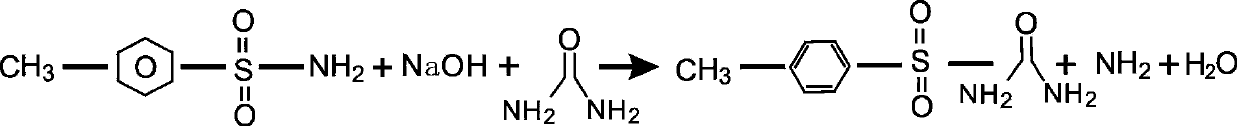
Preparation method of toluenesulfonylurea
A technology of toluenesulfonylurea and p-toluenesulfonamide, applied in the preparation of pharmaceutical intermediate p-toluenesulfonylurea, the important intermediate field of synthesizing sulfonylurea oral hypoglycemic drugs tolbutamide and gliclazide, capable of Solve the problems of slow progress and few reports, and achieve the effects of short reaction cycle, reduced production cost and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Install a 1000ml three-necked bottle and a stirring oil bath heating device. Add 40 g of urea, 20 g of NaOH and 70 g of p-toluenesulfonamide in sequence, and heat up in an oil bath. Heating to above 130°C, the material is partially melted, start stirring, keep warm at 130-135°C, and react for about 100 minutes, the material liquid becomes thick enough to be unable to stir, stop stirring. Vacuum for 1 to 2 minutes to extract the ammonia gas generated by the reaction, and remove the oil bath. Add 700ml of drinking water, stir to dissolve, add 30% dilute sulfuric acid dropwise, adjust pH = 4, filter, rinse the filter cake with drinking water until pH = 5-6, discharge, crush, and dry at 100°C to obtain p-toluene Sulfonylurea 67.1g, purity 99.9%.
Embodiment 2
[0020] Install a 1000ml three-necked bottle and a stirring oil bath heating device. Add 40 g of urea, 20 g of NaOH and 70 g of p-toluenesulfonamide in sequence, and heat up in an oil bath. Heating to above 130°C, the material is partially melted, start stirring, keep warm at 135-140°C, and react for about 120 minutes, the material liquid becomes thick enough to be unable to stir, stop stirring. Vacuum for 1 to 2 minutes to extract the ammonia gas generated by the reaction, and remove the oil bath. Add 700ml of drinking water, stir to dissolve, add 30% dilute sulfuric acid dropwise, adjust pH = 4.5, filter, rinse the filter cake with drinking water until pH = 5-6, discharge, crush, and dry at 100°C to obtain p-toluene Sulfonylurea 66.9g, purity 99.0%.
Embodiment 3
[0022] Install a 1000ml three-necked bottle and a stirring oil bath heating device. Add 40 g of urea, 20 g of NaOH and 70 g of p-toluenesulfonamide in sequence, and heat up in an oil bath. Heat up to 130°C or above, the material is partially melted, start stirring, keep warm at 140-145°C, and react for about 95 minutes, the material liquid becomes thick enough to be unable to stir, stop stirring. Vacuum for 1 to 2 minutes to extract the ammonia gas generated by the reaction, and remove the oil bath. Add 700ml of drinking water, stir to dissolve, add 40% dilute sulfuric acid dropwise, adjust pH=4, filter, rinse the filter cake with drinking water until pH=5~6, discharge, crush, and dry at 100°C to obtain p-toluene Sulfonylurea 66.5g, purity 98.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com