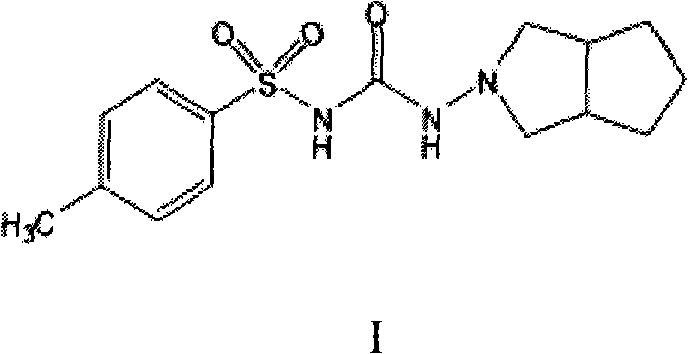
Method for synthesizing gliclazide and intermediate thereof
A synthesis method and compound technology, applied in the preparation of sulfonamides, organic chemistry, etc., can solve problems such as difficult control of reaction conditions, and achieve the effects of easy oxidation, improved product quality, and easy control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
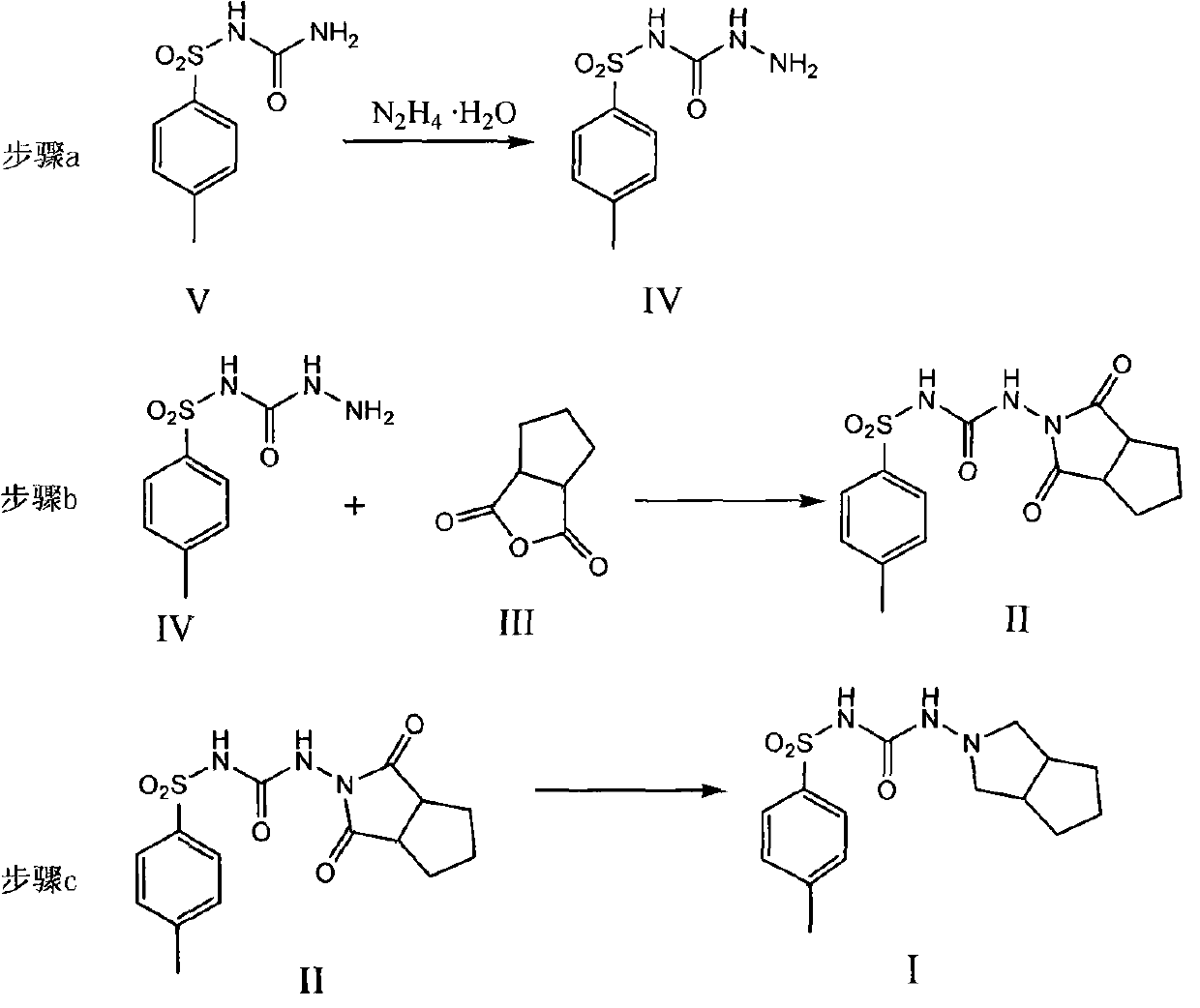
[0029] Add 30g of p-toluenesulfonylurea (compound V), 19.5g of sodium carbonate, 200ml of n-butanol, and 11g of hydrazine hydrate into a 500ml reaction flask. 300ml of water, separated to remove the organic layer, the aqueous layer was adjusted to PH=7 with hydrochloric acid, a large amount of white solids precipitated, filtered with suction, washed with water, and the warm product was dried at 65-70°C for 12 hours to obtain 23g of the target product N-[(4-methylbenzene base) sulfonyl]-hydrazide carboxamide (compound IV), yield 71.6%.
Embodiment 2
[0031] Add p-toluenesulfonylurea (compound V) 30g, sodium carbonate 19.5g, n-butanol 200ml, hydrazine hydrate 11g in the 500ml reaction bottle, add, heat to reflux, keep warm for 12 hours, keep warm, and recover normal by vacuum distillation. After 150ml of butanol, add 300ml of water, distill 50ml under normal pressure, cool to room temperature, adjust PH=7 with hydrochloric acid, a large amount of white solid precipitates, filter with suction, wash with water, and dry the warm product at 65-70°C for 12 hours to obtain 30g of the target product N- [(4-Methylphenyl)sulfonyl]-hydrazide carboxamide (compound IV), yield 93.4%.
Embodiment 3
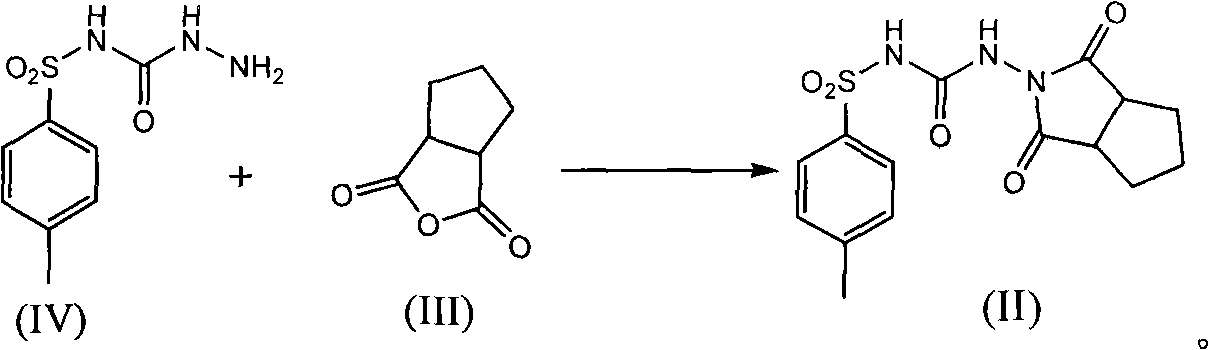
[0032] Example 3: Synthesis of 1-(1,3-dicarbonyl-hexahydrocyclopentadieno[c]pyrrol-2(1H)-yl)-3-p-toluenesulfonylurea (Compound II)
[0033] Add 3.0g p-toluenesulfonic acid, N-[(4-methylphenyl)sulfonyl]-hydrazide carboxamide (compound IV) 19.5g, 1,2-cyclopentane phthalic anhydride in a 500ml reaction flask (Compound III) 15g, 200ml of toluene, after adding, heated to reflux, refluxed and separated water for 12 hours, after separating water, 150ml of toluene was recovered by distillation under reduced pressure and 300ml of water was added, 100ml of hydrated toluene was recovered by normal pressure distillation, cooled to room temperature, Suction filtration, washing with water, drying the warm product at 65-70°C for 12 hours to obtain 18 g of the target product 1-(1,3-dicarbonyl-hexahydrocyclopenta[c]pyrrol-2(1H)-yl)-3- p-toluenesulfonylurea (compound II), yield 60.2%.
[0034] p-toluenesulfonic acid acts as an acid catalyst in this reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com