Gliclazide sustained release tablets and preparation method thereof
A technology for gliclazide and sustained-release tablets, applied in the field of medicine, can solve the problems of unsustainable blood drug concentration of gliclazide, poor sustained-release stability of sustained-release tablets, and inability to exert drug efficacy, etc., so as to ensure drug efficacy. , The effect of stable drug effect and reducing dependence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
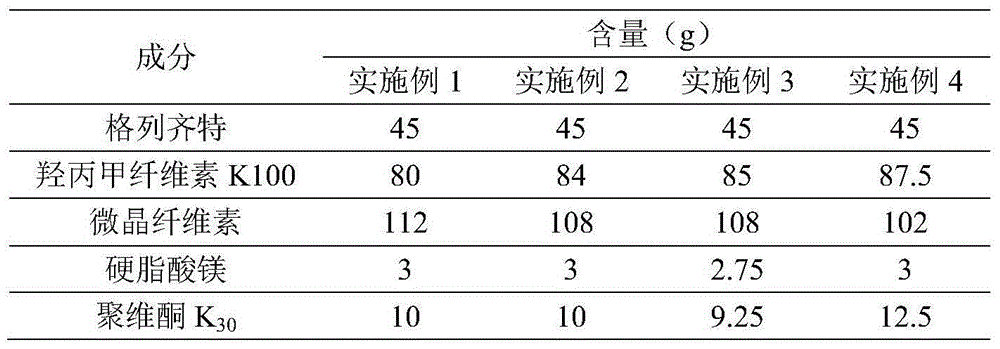
Embodiment 1-4
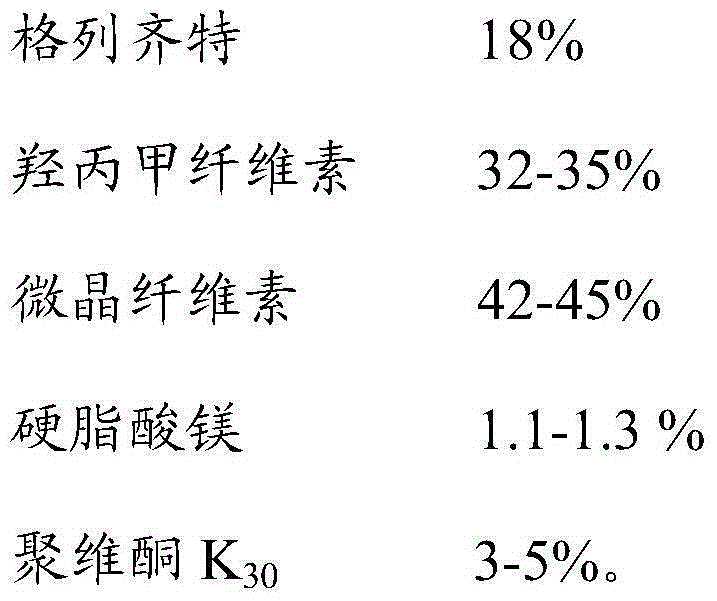
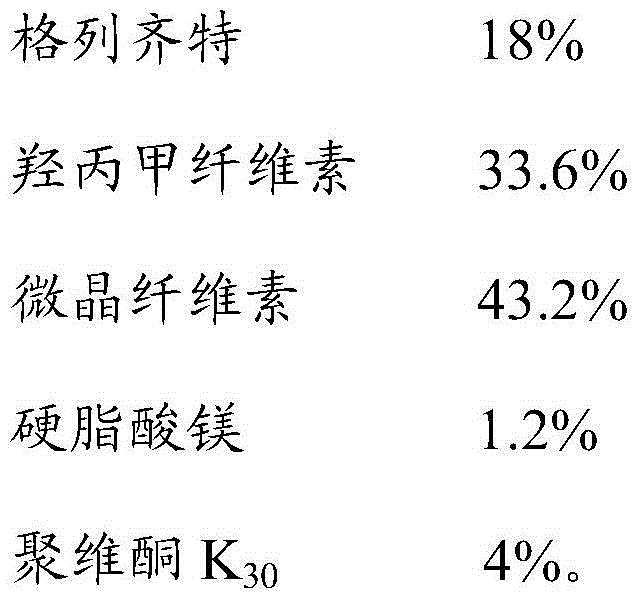
[0028] Gliclazide sustained-release tablets were prepared according to the ratio of raw materials in Table 1 below:
[0029] Table 1. Raw material ratio of gliclazide sustained-release tablets
[0030]
[0031] The preparation method of Gliclazide sustained-release tablet:
[0032] 1) Raw material preparation: pass gliclazide, hypromellose, and microcrystalline cellulose through a 80-mesh sieve, and pass magnesium stearate through a 20-mesh sieve for later use; mix 95% ethanol with povidone K 30 Mix evenly to make 5% povidone K by weight 30 Ethanol solution is used as a wetting agent for standby;
[0033] 2) Mixing and granulation: pour gliclazide, hypromellose and microcrystalline cellulose into a wet mixing granulator, dry mix for 5 minutes, then add a wetting agent, and wet mix for 5 minutes to make granules ;
[0034] 3) Drying: Spread the granules prepared in step 2) evenly on a baking tray with a thickness of no more than 2 cm, and send them to a hot air circulati...
Embodiment 5
[0039] According to the dissolution determination method specified in the second appendix XC of "Chinese Pharmacopoeia" in 2010, the gliclazide sustained-release tablets produced in Examples 1-4 were tested respectively, and the test results were shown in Table 2 below:
[0040] Table 2, Example 1-4 Gliclazide Sustained Release Tablets Dissolution Test Data
[0041]
[0042] Carry out accelerated test to Gliclazide Sustained-release Tablet in embodiment 1-4, measure Gliclazide (C 15 h 21 N 3 o 3 S) content, detection data is as follows table 3:
[0043] Table 3, embodiment 1-4 accelerated test detection data
[0044]
[0045]
[0046] Tables 2 and 3 show that the gliclazide sustained-release tablet of the present invention prepared by the present invention has stable components, good sustained-release effect, and can continuously maintain the blood concentration of gliclazide in the body above the effective therapeutic concentration, ensuring long-lasting drug con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com