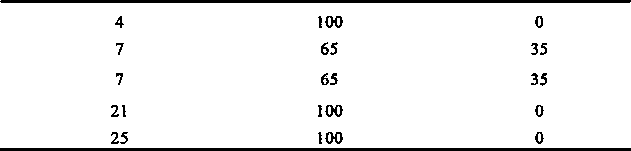Pharmaceutical composition for treating hypertension and preparation method of pharmaceutical composition
A pharmacy and reserpine technology, which is applied in the direction of drug combination, pharmaceutical formula, and active ingredients of heterocyclic compounds, etc., can solve problems such as the instability of dihydralazine sulfate and vitamin B1, and achieve the effect of stable components and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Prescription: 1000 tablets
[0078]
[0079]
[0080] Preparation Process:
[0081] 1. Weigh an appropriate amount of HPC (hydroxypropyl cellulose), add water to make a 5% HPC solution for later use;
[0082] 2. Take the prescribed amount of vitamin B1 and vitamin B6, add 10g of starch and 20g of pregelatinized starch, granulate with 95% ethanol, dry at 50°C, and sieve through an 80-mesh sieve;
[0083] 3. Use the above-mentioned HPC solution to carry out bottom spray coating on the above-mentioned granules, increase the weight by 40%, and obtain the granules (1);
[0084] 4. Take the remaining prescription amount of raw and auxiliary materials, granulate with 95% ethanol, sieve with 14 mesh to make soft material, dry at 50°C, and granulate with 14 mesh sieve to obtain granules (2);
[0085] 5. Mix the granules (1) and granules (2) into tablets;
[0086] 6. Opadry coating powder is added with water to make a 12% coating solution, and the core coating weight is...
Embodiment 2
[0094] Prescription: 1000 tablets
[0095]
[0096]
[0097] Preparation Process:
[0098] 1. Take an appropriate amount of HPC and add water to make a 5% solution, spray powder coating on the bottom of vitamin B1, take it out after a weight gain of 30%, and screen out 80-100 mesh particles;
[0099] 2. Take the raw and auxiliary materials of the prescription amount except vitamin B1, add the above-mentioned vitamin B1 granules, use 95% ethanol to granulate, make soft materials with a 14-mesh sieve, dry at 50°C, granulate with a 14-mesh sieve, and press into tablets;
[0100] 3. Opadry coating powder is added with water to make a 12% coating solution, and the weight of the tablet core coating is increased by 4%.
[0101] The prepared compound reserpine tablets are detected according to quality standards, and the results are as follows:
[0102]
[0103] The test results of influencing factors are shown in the table below:
[0104]
[0105] The accelerated stabi...
Embodiment 3
[0108] Prescription: 1000 tablets
[0109]
[0110] Preparation Process:
[0111] Preparation Process:
[0112] 1. Take an appropriate amount of EC plus 95% ethanol solution to prepare a 6% EC solution, spray powder coating on the bottom of vitamin B1, take it out after a weight gain of 30%, and screen out 80-100 mesh particles;
[0113] 2. Take the raw and auxiliary materials of the prescription amount except vitamin B1, add the above-mentioned vitamin B1 granules, use 95% ethanol to granulate, make soft materials with a 14-mesh sieve, dry at 50°C, granulate with a 14-mesh sieve, and press into tablets;
[0114] 3. Opadry coating powder is added with water to make a 12% coating solution, and the weight of the tablet core coating is increased by 4%.
[0115] The prepared compound reserpine tablets are detected according to quality standards, and the results are as follows:
[0116]
[0117] The test results of influencing factors are shown in the table below:
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com