HPLC-MS/MS (High Performance Liquid Chromatography-Mass Spectrum/Mass Spectrum) technique-based method for detecting blood concentration of NMDA (N Methyl D Aspartate) receptor antagonist JCC-02
A JCC-02, receptor antagonist technology, applied in measurement devices, instruments, scientific instruments, etc., can solve the problem of undiscovered pharmacokinetic characteristics, and achieve the effects of short measurement time, time saving, and prevention of matrix effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1 methodological research
[0046] 1. Instruments and equipment:
[0047] Agilent 1290 Infinity high performance liquid chromatograph, American Agilent company;
[0048] AB API4000 triple quadrupole tandem mass spectrometer, AB Sciex, USA;
[0049] D3024R centrifuge, Beijing Dalong;
[0050] XW-80A vortex mixer, Shanghai Qingpu Huxi Instrument Factory;
[0051] CPA 124S electronic analytical balance, Germany Sartorius company;
[0052] Weight scale, Cixi Tiandong Weighing Apparatus Factory;
[0053] 2. Reagents and reagents:
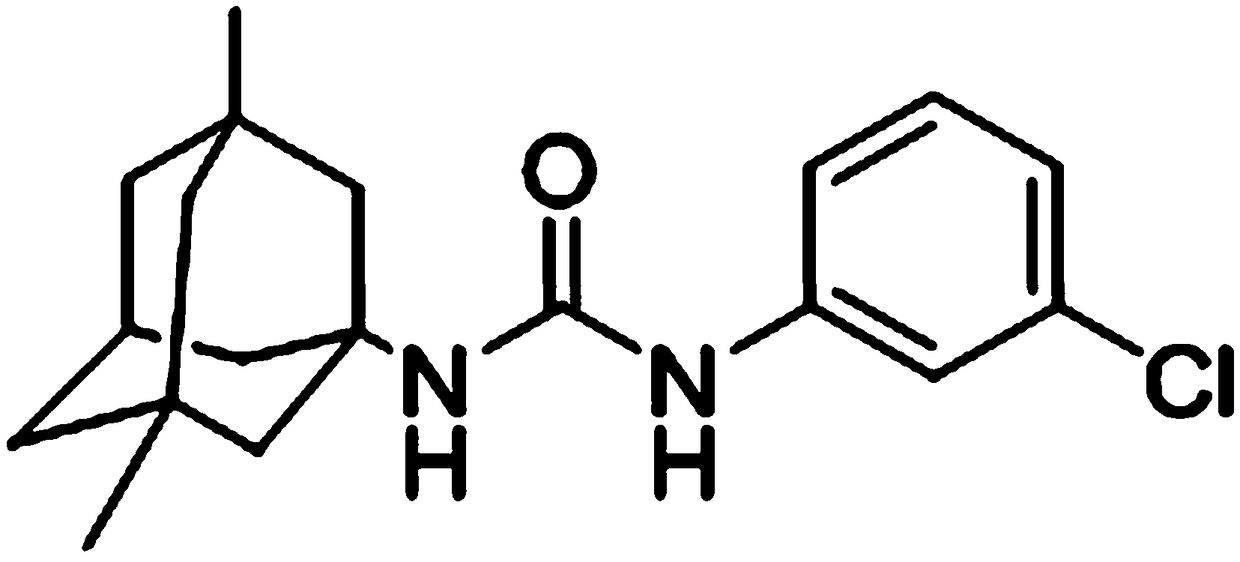
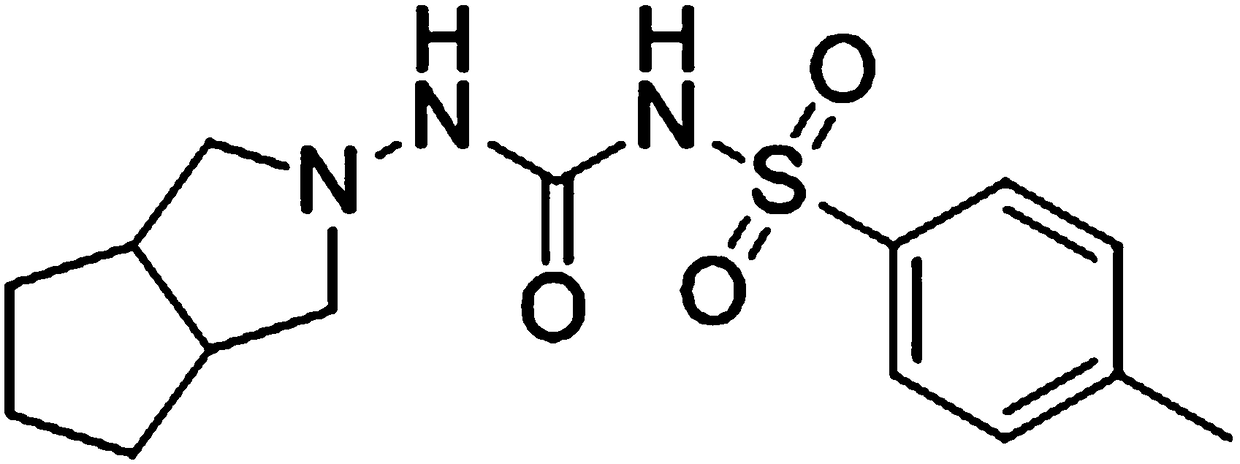
[0054] JCC-02 (≥purity 98.0%), provided by the Department of Medicinal Chemistry, Shenyang Pharmaceutical University;
[0055] Gliclazide (purity 99.9%), China National Institute for the Control of Pharmaceutical and Biological Products;
[0056] Methanol (chromatographically pure), Dima Technology Co., Ltd.;
[0057] Acetonitrile (chromatographically pure), Thermo Fisher Scientific Co., Ltd.;
[0058] Formic acid (chromatograp...
example 2
[0090] Example 2 Pharmacokinetic Research
[0091] 1. Dosing regimen and sample collection
[0092] Accurately weigh 3.5 mg of JCC-02 sample, add 50 μL DMSO to dissolve, then add 500 μL Tween-80, and mix well by ultrasonic. Continue to add 2.5mL PEG-400, and ultrasonically mix again. Dilute to 50mL with pure water, mix by ultrasonic to prepare 0.07mg·mL -1 JCC-02 clarification solution.
[0093] Before the experiment, SD rats were fasted for 12 hours and had free access to water. Weigh and label 8 SD rats (4 females and 4 males) weighing 200±20g, according to 0.7mg·kg -1 The dosage is administered by intragastric administration. At the designated blood collection time points (0, 0.17, 0.33, 0.5, 0.75, 1, 1.5, 2.5, 4.5, 6.5, 9, 12 and 24 hours), blood was collected from the orbital venous plexus, and the blood volume was 0.4 mL. 1.5mL heparinized centrifuge tube. at 10000r·min -1 Centrifuge at a high speed for 10 minutes, transfer the upper layer of plasma to an empty c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com