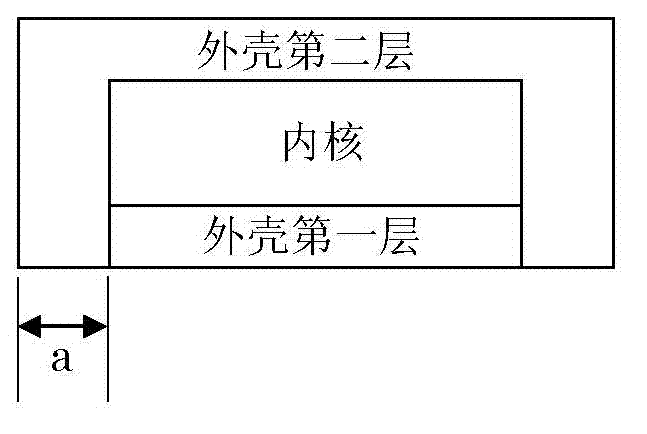
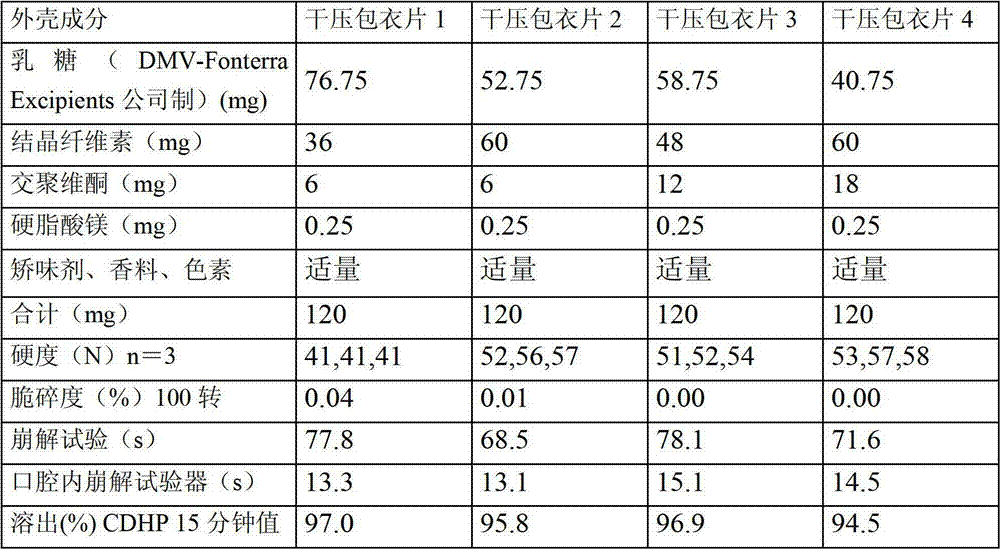
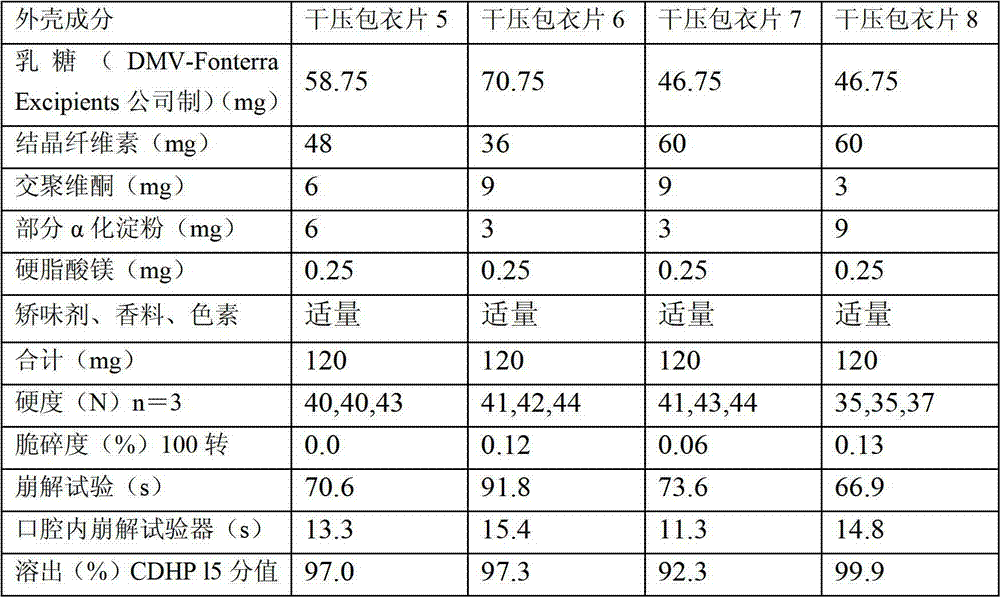
Dry pressed coating tablet containing tegafur, gimeracil and oteracil potassium
A technology of dry-pressed coated tablets and potassium oxonate, which is applied to medical preparations containing active ingredients, medical preparations with non-active ingredients, organic active ingredients, etc., can solve the problems of pharmaceutical exposure of medical workers, and achieve Tablet Strength Rapid, Reduced Exposure, Reduced Risk Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0065] Hereinafter, the present invention will be described in more detail with reference examples, working examples, and test examples, but the present invention is not limited to these examples. It should be noted that the tegafur and gimeracil used in this example were manufactured by Dapeng Pharmaceutical Industry Co., Ltd., and the potassium oxonate was manufactured by Sumitomo Chemical Industry Co., Ltd. In addition, hydroxypropyl cellulose (HPC) is made by Nippon Soda Co., partially α-starch and crystalline cellulose are made by Asahi Kasei Chemical Co., Ltd., magnesium stearate is made by Taiping Chemical Co., Ltd., and crospovidone is made by BASF Incorporated.
reference example 1
[0066] Reference Example 1 (Preparation of Core Component 1)
[0067] Using a fluidized bed granulator Multiplex MP-01 (manufactured by Powrex), in a mixture of 66.1 g of tegafur, 19.15 g of gimeracil, 64.75 g of potassium oxonate, and 150 g of lactose (manufactured by Borculo Domo Ingredients), 200.0 g of water was sprayed and granulated to prepare a core component.
reference example 2
[0068] Reference Example 2 (Preparation of Core Component 2)
[0069] By the same method as in Reference Example 1, in a mixture of 66.1 g of tegafur, 19.15 g of gemerazepam, 64.75 g of oxonate potassium, and 150 g of lactose (manufactured by Borculo Domo Ingredients Co., Ltd.), 199.0 g of water was dissolved with The liquid obtained by HPC 1.0 g was sprayed and granulated to prepare a core component.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com