Use of tanshinone in resisting uric acid nephropathy
A technology of uric acid nephropathy and tanshinone, applied in the field of natural medicines, can solve problems such as unclear structure of active ingredients, uncertain stability and reproducibility of quality control pharmacological experiment results, unclear synergistic effect, etc., and achieve good kidney protection , reduce blood uric acid, good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of tanshinone compounds and quantification of the four main components of tanshinone II A, 15,16-dihydrotanshinone I, cryptotanshinone and tanshinone.
[0033] 1 Materials and Instruments
[0034] The extraction and separation reagent ethyl acetate (Nanjing Chemical Reagents) was analytically pure, and the identification reagents methanol (Shanghai Xingkegao) and acetonitrile (Merck) were chromatographically pure. Salvia miltiorrhiza was purchased from Shandong Rizhao.
[0035] Shimadzu liquid chromatography (Shimadzu Corporation), rotary evaporator EYELA OSB-2100 (Nanjing Huiheng).
[0036] 2 methods
[0037] 2.1 Extraction and separation
[0038] Weigh 150g of Salvia miltiorrhiza powder, put it into a stoppered Erlenmeyer flask, add 8 times the amount of ethyl acetate for ultrasonic extraction for 2 hours. The extracted obtained filtrate and filter residue were obtained by suction filtration, the filtrates were combined, concentrated by rotar...
Embodiment 2
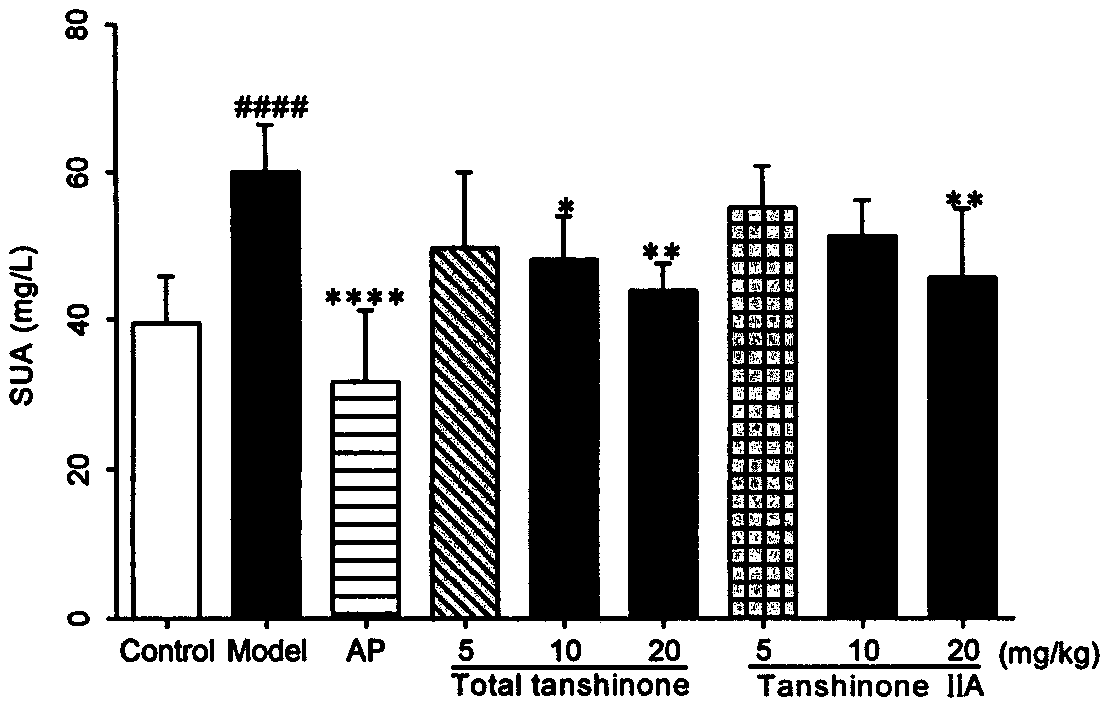
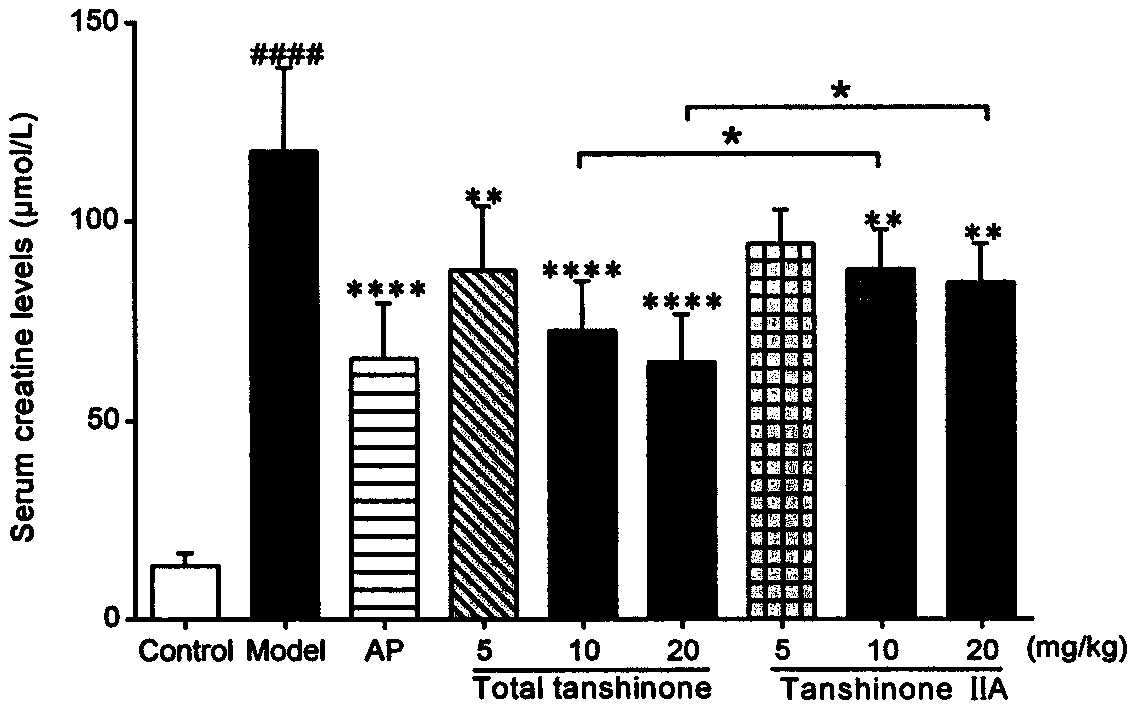
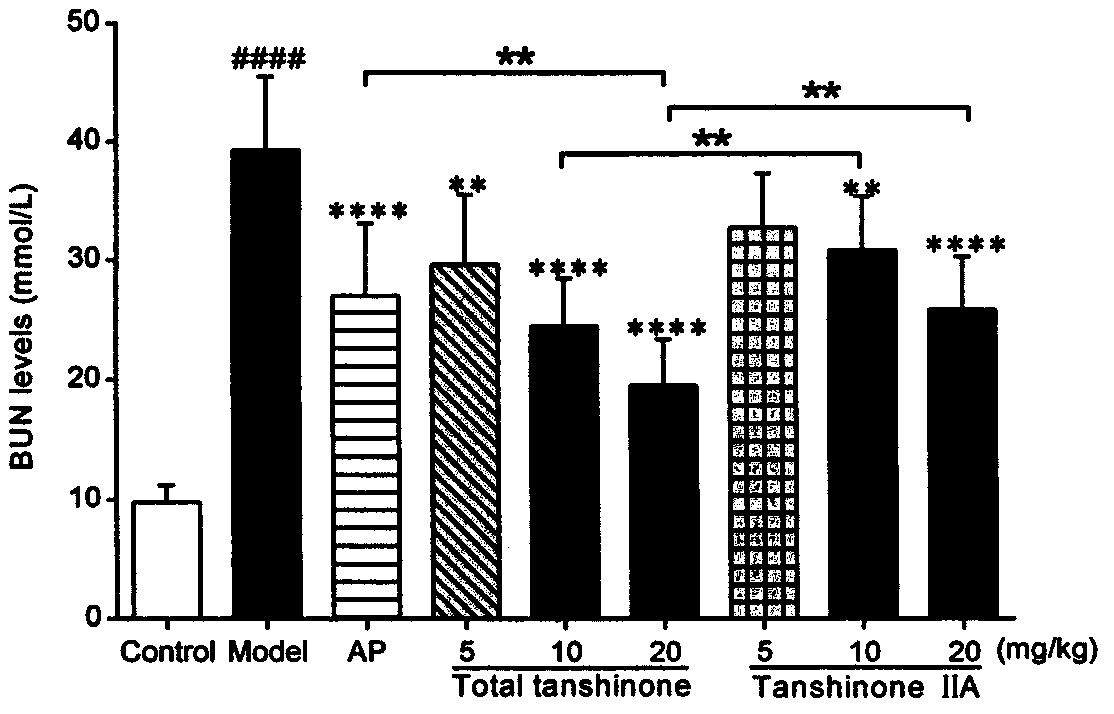
[0062] Example 2 Detection of general indicators and biochemical indicators of the effects of tanshinone compounds and tanshinone II A monomers on mice with uric acid nephropathy.
[0063] 1 animal
[0064] SPF-grade Kunming mice were purchased from the Experimental Animal Center of the Academy of Military Medical Sciences of the Chinese People’s Liberation Army. After a week of adaptive feeding in an environment with a temperature of 22±3°C and a humidity of 65±5%, the experiment began. The mice had free access to food and water. .
[0065] 2 Materials and Instruments
[0066] Uric acid, creatinine, urea nitrogen test kit (Nanjing Jiancheng Bioengineering Research Institute).
[0067] Microplate reader (Beijing Hao Nuosi Technology Co., Ltd., Beijing Hao Nuosi Technology Co., Ltd., varioskanflash), electric constant temperature water bath (Guohua Electric Co., Ltd., HH-4 digital display constant temperature water bath), centrifuge (Thermo Corporation of the United States ,...
Embodiment 3
[0108] The preferred process of embodiment 3 four kinds of compound ratios in described composition and effect thereof
[0109] The ratio of the four compounds of tanshinone II A, 15,16-dihydrotanshinone I, cryptotanshinone and tanshinone in the composition is 6:3:6:4.
[0110] 1 animal
[0111] SPF-grade Kunming mice were purchased from the Experimental Animal Center of the Academy of Military Medical Sciences of the Chinese People’s Liberation Army. After a week of adaptive feeding in an environment with a temperature of 22±3°C and a humidity of 65±5%, the experiment began. The mice had free access to food and water. .
[0112] 2 Materials and Instruments
[0113] Tanshinone II A, 15,16-dihydrotanshinone I, cryptotanshinone, and tanshinone monomer compounds are all purchased from outside.
[0114] Uric acid, creatinine, urea nitrogen test kit (Nanjing Jiancheng Bioengineering Research Institute).
[0115] Microplate reader (Beijing Hao Nuosi Technology Co., Ltd., Beijing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com