Oral preparation containing tegafur, gimeracil and oteracil potassium
An oral preparation, the technology of tegafur, which is applied in the field of pharmaceutical preparations, can solve the problems of inconvenient industrial production, complex production process, and large gastrointestinal irritation, and achieve the goals of improving medication compliance, simple preparation process, and reducing irritation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-21
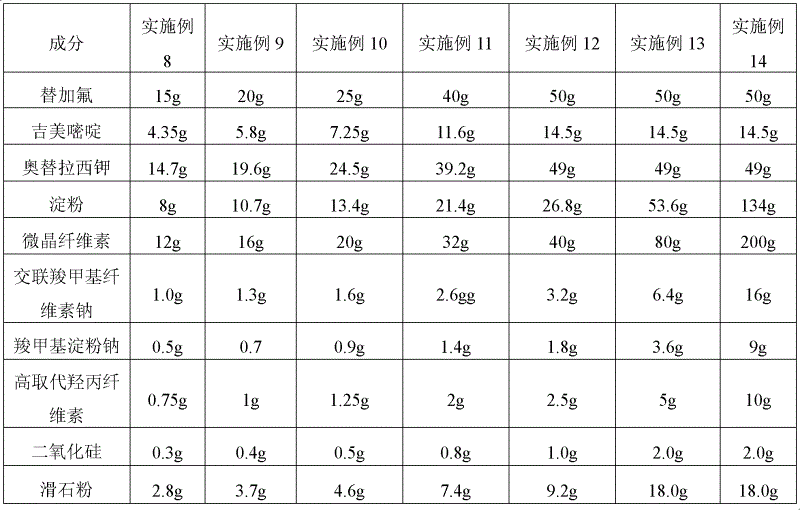
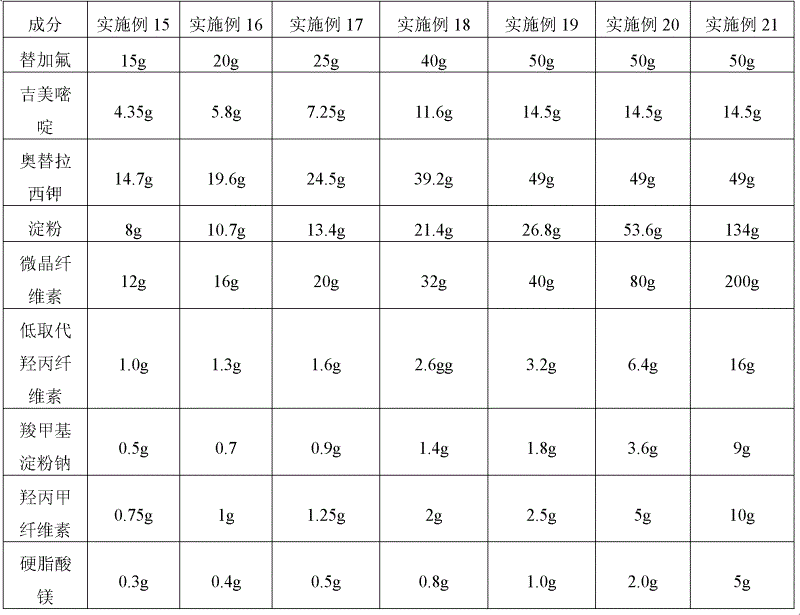
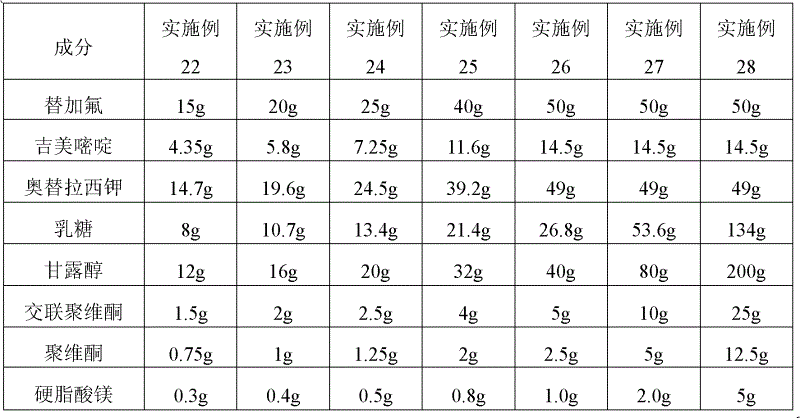
[0035] The particle sizes of the active ingredients are: 80μm≤tegafur≤180μm, 50μm≤gimeracil≤150μm, 50μm≤octeracil potassium≤150μm, and the raw material composition of the oral preparation is shown in Table 1-3.
[0036] Table 1. Examples 1-7 Oral solid preparations of tegafur, gimeracil, and oteracil potassium
[0037] Element
Example 1
Example 2
Example 3
Example 4
Example 5
Example 6
Example 7
15g
20g
25g
40g
50g
50g
50g
Gimeracil
4.35g
5.8g
7.25g
11.6g
14.5g
14.5g
14.5g
14.7g
19.6g
24.5g
39.2g
49g
49g
49g
8g
10.7g
13.4g
21.4g
26.8g
53.6g
134g
12g
16g
20g
32g
40g
80g
200g
Crospovidone
1.5g
2g
2.5g
4g
5g
10g
...
Embodiment 22-42
[0065] The particle sizes of the active ingredients are: 20μm≤tegafur≤80μm, 3μm≤gimeracil≤50μm, 3μm≤octeracil potassium≤50μm, and the composition of raw materials for oral preparations is shown in Table 4-6.
[0066] Table 4. Preparation of oral solid preparations of tegafur, gimeracil, and oteracil potassium
[0067]
[0068] Preparation Process
[0069] 1) Ingredients: Weigh lactose, tegafur, gimeracil, oteracil potassium, crospovidone, mannitol, povidone, and magnesium stearate according to the prescription. The povidone is made into 10wt% aqueous solution or water-ethanol (1:1) mixed solution for later use.
[0070] 2) Premixing: add lactose, tegafur, gimeracil, oteracil potassium, crospovidone, and mannitol to the HLSH2-6A wet granulator in sequence. The premixing process parameters are the same as in Examples 1-7.
[0071] 3) Making soft material: the process parameters are the same as those in Examples 1-7, turn on the equipment, and add 10 wt% povidone aqueous so...
Embodiment 43-63
[0091] Preparation of oral preparations with particle sizes of active ingredients of tegafur ≤ 20 μm, gimeracil ≤ 3 μm, and oteracil potassium ≤ 3 μm
[0092] Table 7. Oral solid preparations of tegafur, gimeracil, and oteracil potassium in Examples 43-49
[0093]
[0094] The preparation process is as follows:
[0095] 1) Ingredients: Weigh lactose, tegafur, gimeracil, oteracil potassium, crospovidone, mannitol, povidone, and magnesium stearate according to the prescription.
[0096] 2) Premixing: add lactose, tegafur, gimeracil, oteracil potassium, crospovidone, povidone, and mannitol to the HLSH2-6A wet granulator in sequence. The premixing process parameters are the same as in Examples 1-7.
[0097] 3) Making soft material: Set the process parameters as in Example 1-7, turn on the equipment, add water or a water-ethanol (1:1) mixed solution in a stirring state to make a suitable soft material.
[0098] 4) ~ 8) wet granulation, drying, dry granulation, total blending,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com