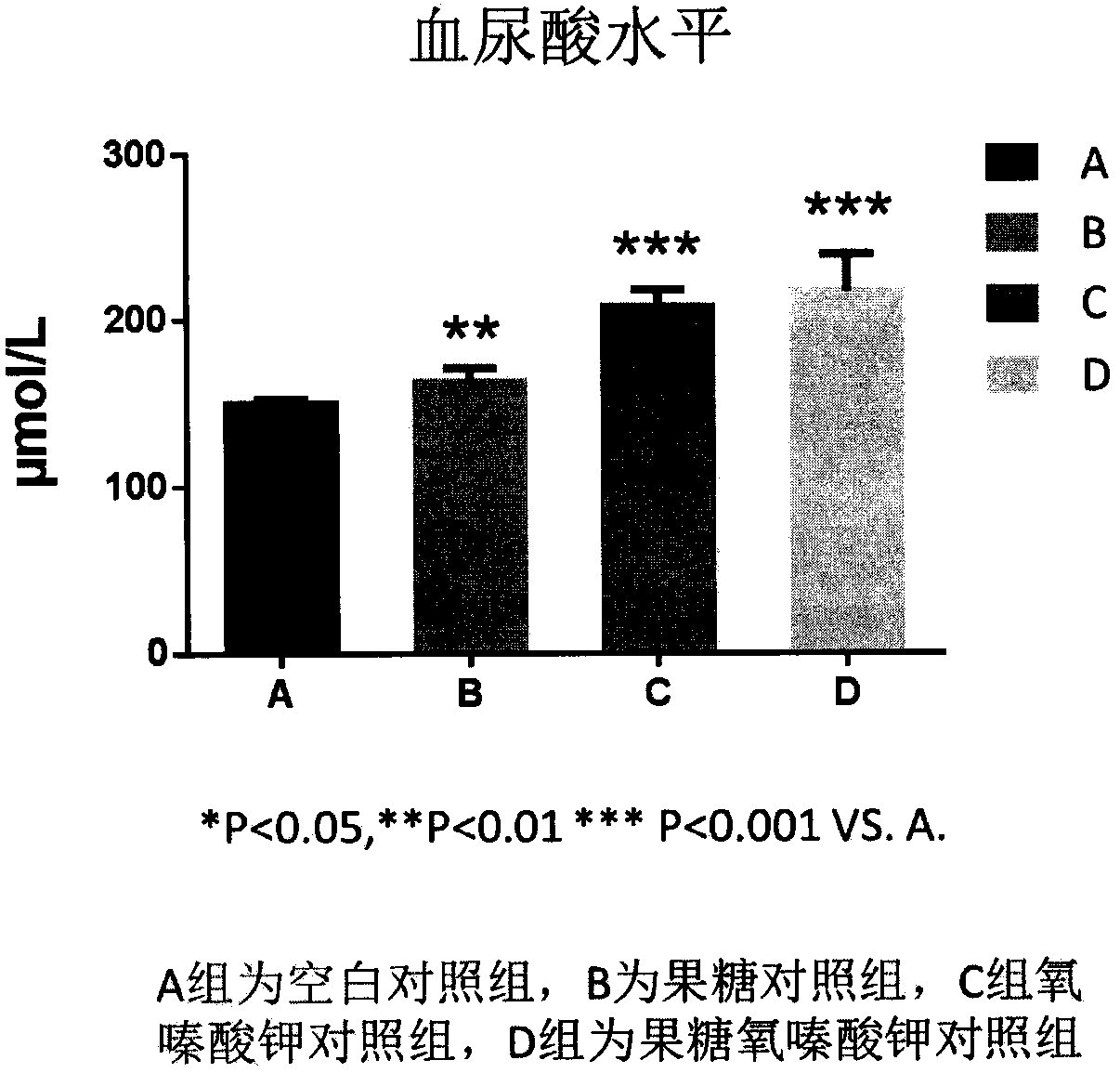
Duplication method of rat continuous hyperuricemia model
A technology of hyperuricemia and model, which can be used in medical preparations containing active ingredients, pharmaceutical formulations, organic active ingredients, etc., can solve problems such as abnormality, and achieve good application prospects and clinical significance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1: The specific steps of the specific construction of hyperuricemia model in this embodiment are as follows
[0015] 1) Animal selection and model construction: Select 8-week-old male clean-grade SD rats, weighing 180-220g, provided by Shanghai Xipuer-Bikay Experimental Materials Co., Ltd., and bred in the clean-grade animal breeding room of China Pharmaceutical University. During the period, the room temperature was controlled at about 22±2°C, the humidity was controlled at about 55±5°C, the lighting was alternated 12h / 12h, and the rats were free to eat and drink water to observe the changes in the body characteristics of the rats, and 20 SD rats with normal body characteristics were selected for modeling experiment.
[0016] 2) Materials and equipment:
[0017] Potassium oxonate (CAS: 2207-75-2) was purchased from Shanghai Shifeng Biotechnology Co., Ltd., D-fructose (CAS: 57-4-7) was purchased from Biosharp Company; uric acid assay kit was purchased from N...
Embodiment 2
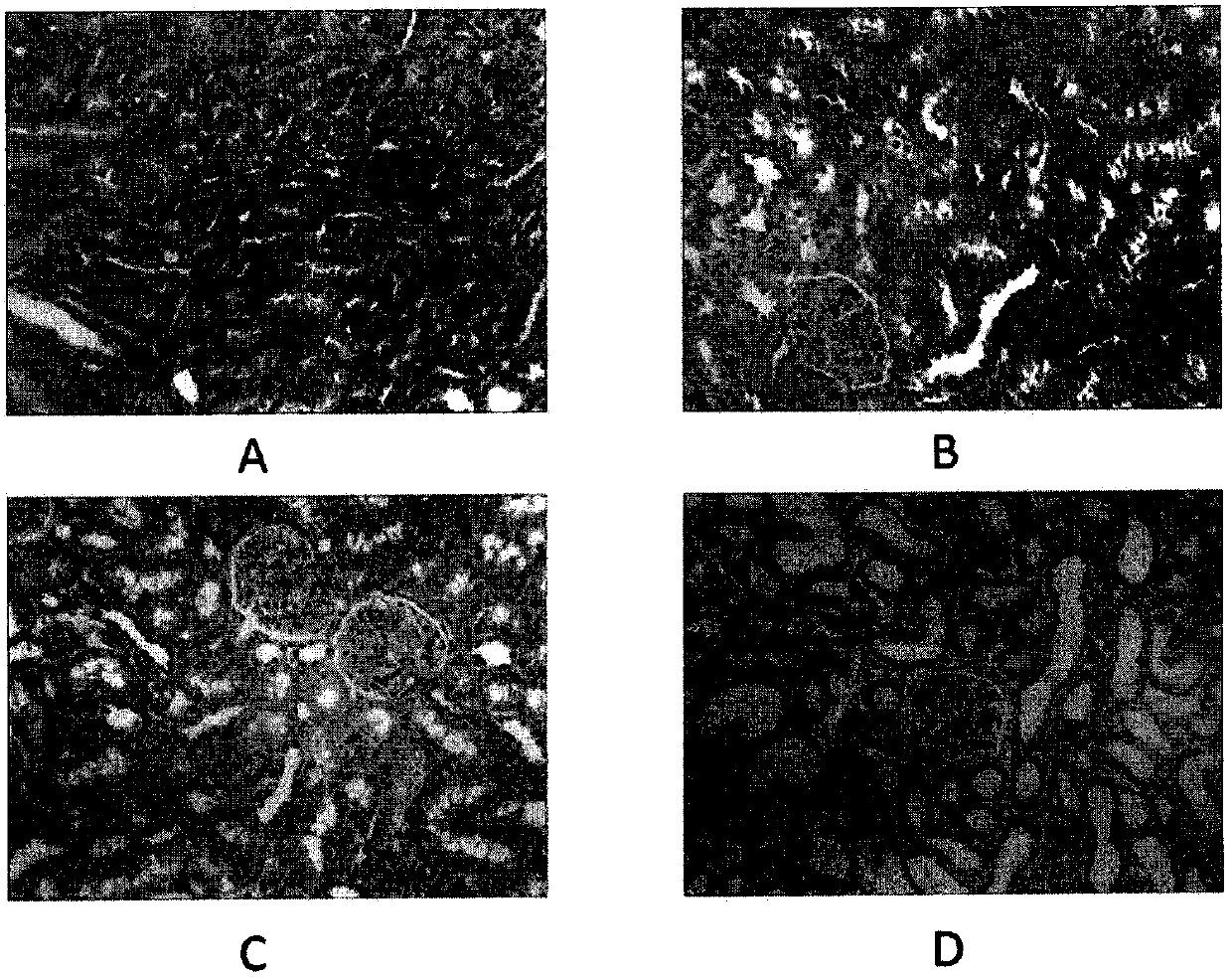
[0019] Embodiment 2: This embodiment uses the animal intervention of Embodiment 1 to carry out the experiment. At the end of the third week of the animal experiment, the rats were fasted for 12 hours and then sacrificed. After the sacrifice, the abdominal cavity was quickly opened, the right kidney was removed, and placed in 4% paraformaldehyde Fixed in the solution, routinely embedded in paraffin, made 3 μm thick sections for HE staining, and observed under a light microscope (×200); the results are shown in the attached figure, the blank control group was glomeruli and renal tubules with normal structure, Glomeruli were normal in size and evenly distributed, and there was no swelling and degeneration of renal tubules. A certain degree of cytoplasmic separation appeared in the renal tubules of the fructose control group. Obvious vacuoles appeared in the renal tubules of the oxonate potassium control group. In the oxonate potassium fructose group, glomerular atrophy and degen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com