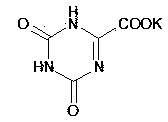
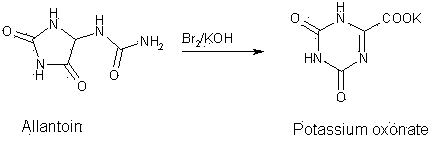
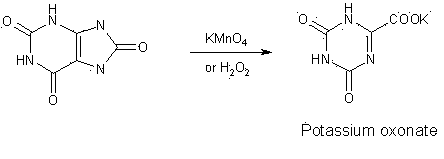
Synthesis technology for preparing oteracil potassium
A technology of potassium oxonate and potassium carbonate, which is applied in the field of synthesis of the pharmaceutical compound potassium oxonate, can solve problems such as the toxicity of the oxidant bromine and large environmental pollution, and achieve the effects of stable quality, high yield, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1. Synthesis of Crude Potassium Oxonate
[0022] Add 1080 mL of distilled water to a 2L reaction flask, and add 275.0 g (4.00 mol) of potassium hydroxide in batches under stirring to obtain a 16.6% (w / w) potassium hydroxide solution. Cool in an ice bath to 5-10°C, add 79.0g (0.50 mol) of allantoin and 4.0g (0.024mol) of potassium iodide (keep the internal temperature ≤ 10°C), stir to dissolve most of it. Cool in an ice bath to 2-5°C, add 420.0 g of sodium hypochlorite solution (10% available chlorine) dropwise under stirring for about 4-5 hours. After dropping, keep the ice bath for 2h, and slowly rise to room temperature (about 2-3h). Stir at room temperature (20°C) for 8h. The end point of the reaction was detected by HPLC (chromatographic column: octadecylsilane bonded silica gel column as filler; mobile phase: methanol: water: phosphoric acid (5:95:0.1); retention time of allantoin was about 2.4min, oxazine The retention time of potassium acid potassium is about ...
Embodiment 2
[0032] Add 1200mL of distilled water into a 2L reaction flask, and add 275.0g (4.00mol) of potassium hydroxide in batches under stirring. Cool in an ice bath to 5-10°C, add 79.0g (0.50mol) of allantoin and 4.0g (0.024mol) of potassium iodide (keep the internal temperature ≤ 10°C), stir to dissolve most of it. Cool in an ice bath to 2-5°C, add 450.0 g of potassium hypochlorite solution (5% available chlorine) dropwise under stirring for about 4-5 hours. After dropping, keep the ice bath for 2h, and slowly rise to room temperature (about 2-3h). Stir at room temperature (20°C) for 12h. A small amount of insoluble matter in the reaction solution was filtered off to obtain a light yellow solution. The inner temperature of the ice bath is 10-12°C, acidify by adding 10% citric acid dropwise under stirring until the pH of the solution is 7, and a large amount of white precipitates are precipitated. Ice bath at 5°C, stirring for 2h. Filter with suction and wash the filter cake with...
Embodiment 3
[0034] Add 1200mL of distilled water into a 2L reaction flask, and add 275.0g (4.00mol) of potassium hydroxide in batches under stirring. Cool in an ice bath to 5-10°C, add 79.0g (0.50mol) of allantoin and 4.0g (0.024mol) of potassium iodide (keep the internal temperature ≤ 10°C), stir to dissolve most of it. Cool in an ice bath to 2-5°C, add 480.0 g of potassium hypobromite solution (5% available bromine) dropwise under stirring for about 4-5 hours. After dropping, keep the ice bath for 2h, and slowly rise to room temperature (about 2-3h). Stir at room temperature (20°C) for 8h. A small amount of insoluble matter in the reaction solution was filtered off to obtain a light yellow solution. The inner temperature of the ice bath is 10-12°C, acidify by adding 10% citric acid dropwise under stirring until the pH of the solution is 7, and a large amount of white precipitates are precipitated. Ice bath at 5°C, stirring for 2h. Filter with suction and wash the filter cake with di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com