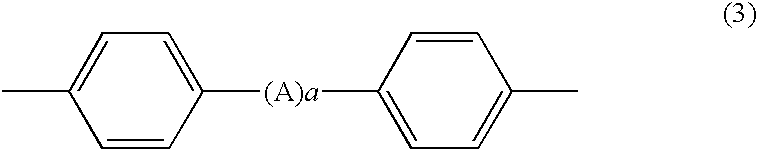
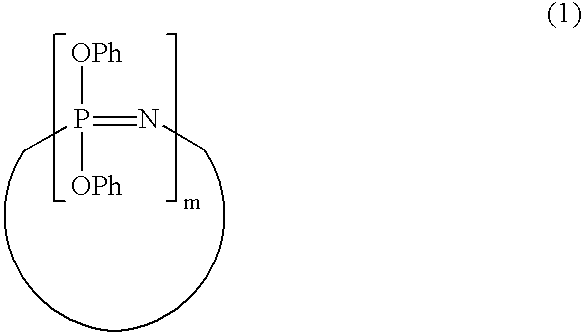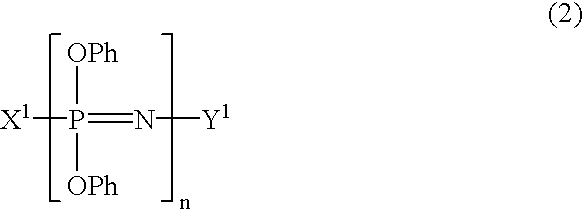Process for producing phenoxyphosphazene compound, flame- retardant resin composition, and flame-retardant resin molding
a technology of phenoxyphosphazene and resin, which is applied in the direction of organic chemistry, chemistry apparatus and processes, group 5/15 element organic compounds, etc., can solve the problems of discoloration of synthetic resins, deterioration of heat resistance, and serious drawbacks of phenoxyphosphazene compounds, so as to reduce mechanical characteristics, reduce molecular weight, and change the hue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Synthesis of phenoxyphosphazene compound having p-phenylene-crosslinked structure
[0136] A solvent-free melt was prepared in the same manner as in Example 1. The melt was then dissolved in a mixed solvent of 1.0 liter of tetrahydrofuran and 0.1 liter of methanol. After addition of 21 g of sodium borohydride, the mixture was stirred at room temperature for 24 hours.
[0137] After the reaction was completed, the reaction mixture was neutralized with 2.5% hydrochloric acid and the organic layer was concentrated under reduced pressure. The solid was dissolved in 1.0 liter of toluene and washed twice with a 2.5% aqueous solution of sodium hydroxide, once with 2.5% hydrochloric acid, once with a 5% aqueous solution of sodium hydrogencarbonate, and twice with a 2% aqueous solution of sodium sulfate. The organic layer was concentrated under reduced pressure. The resulting product was heated and vacuum dried at 80.degree. C. at a pressure of 4 hPa or less for 11 hours, giving 202 g of a white s...
example 3
Synthesis of phenoxyphosphazene compound having 2,2-bis(p-oxyphenyl)isopro-pyridene-crosslinked structure
[0151] 65.9 g (0.7 moles) of phenol and 500 ml of toluene were placed in a 1-liter, 4-necked flask. While stirring the mixture and maintaining the internal temperature at 25.degree. C., 14.9 g (0.65 gram atoms) of finely cut metallic sodium were added. After completion of the addition, stirring was continued for 8 hours at 77.degree. C. to 113.degree. C. until the metallic sodium was completely consumed.
[0152] In parallel with the above reaction, 57.1 g (0.25 moles) of bisphenol-A, 103.5 g (1.1 moles) of phenol and 800 ml of THF were placed in a 3-liter, 4-necked flask. While stirring the mixture and maintaining the internal temperature at 25.degree. C., 11.1 g (1.6 gram atoms) of finely cut metallic lithium were added. After completion of the addition, stirring was continued for 8 hours at 61.degree. C. to 68.degree. C. until the metallic lithium was completely consumed. While s...
example 4
Synthesis of phenoxyphosphazene compound having 2,2-bis(p-oxyphenyl)isopro-pyridene-crosslinked structure
[0159] A phenoxyphosphazene compound was prepared in the same manner as in Example 3 except for the following differences in the production process:
[0160] (a) after completion of the reaction, the reaction mixture was concentrated to remove THF and 1 liter of toluene was added;
[0161] (b) the toluene solution was then washed three times with 0.5 liters of a 5% aqueous solution of sodium thiosulfate and three times with a 2% aqueous solution of sodium sulfate and the organic layer was concentrated under reduced pressure; and
[0162] (c) the resulting product was heated and vacuum dried at 80.degree. C. at a pressure of 4 hPa or less for 11 hours. As a result, 220 g of a white solid was obtained
[0163] The crosslinked phenoxyphosphazene compound thus obtained had an acid value of 0.008 mgKOH / g and a hydrolyzable chlorine content of 0.03%. The final product had a composition of [N.dbd.P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com