Oteracil potassium preparation method
A technology of oteracil potassium and potassium iodide, which is applied in the field of drug synthesis, can solve the problems of high bromine toxicity, potential safety hazards, and large pollution, and achieve the effects of high product yield, stable quality, and simple raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 oteracil potassium
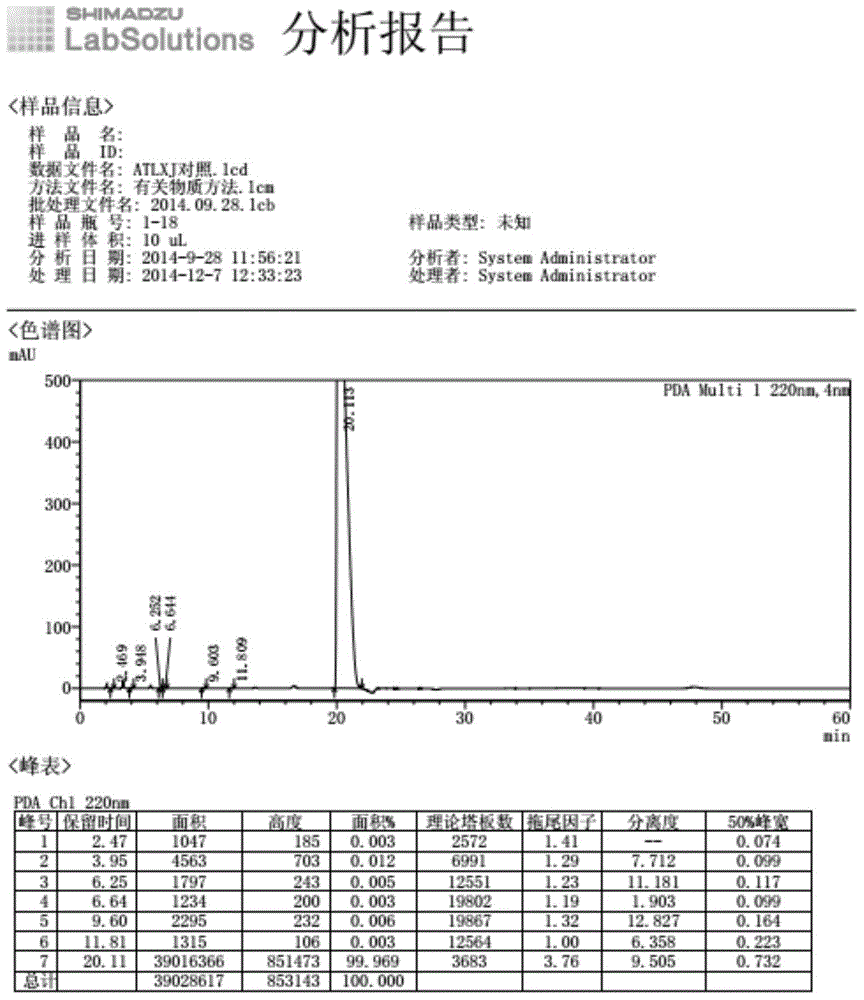
[0033] At room temperature, dissolve 336g potassium hydroxide (6mol) in 1200ml purified water, add 4.8g potassium iodide (0.03mol), add 95g allantoin (0.6mol), stir for 30min, add potassium persulfate 33g (0.12mol) in batches , stirred at room temperature for 24 hours until the reaction of the raw materials was complete, filtered off the insoluble matter, adjusted the pH of the filtrate to 6 with sulfuric acid, precipitated a solid, and dried to obtain 312 g of the product, with a purity of 99.8% and a maximum of 0.05%.
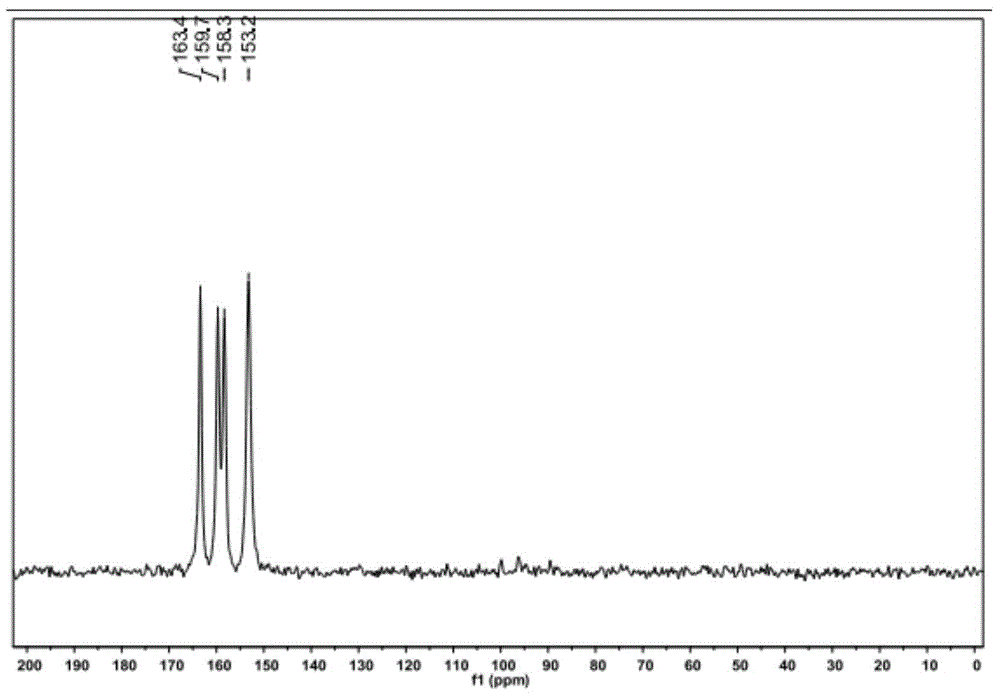
[0034] The solid-state nuclear magnetic resonance and HPLC analysis of gained product:
[0035] Test method: solid-state nuclear magnetic resonance is tested with a 500MHz nuclear magnetic resonance spectrometer. Data acquisition conditions: 13C CP / MAS NMR, 8kHz rotational speed, adamantane calibration (29.5ppm), contact time 2ms, pulse delay 8s. Timing 1h.
[0036] The solid-state NMR spectrum of...
Embodiment 2
[0037] The preparation of embodiment 2 oteracil potassium
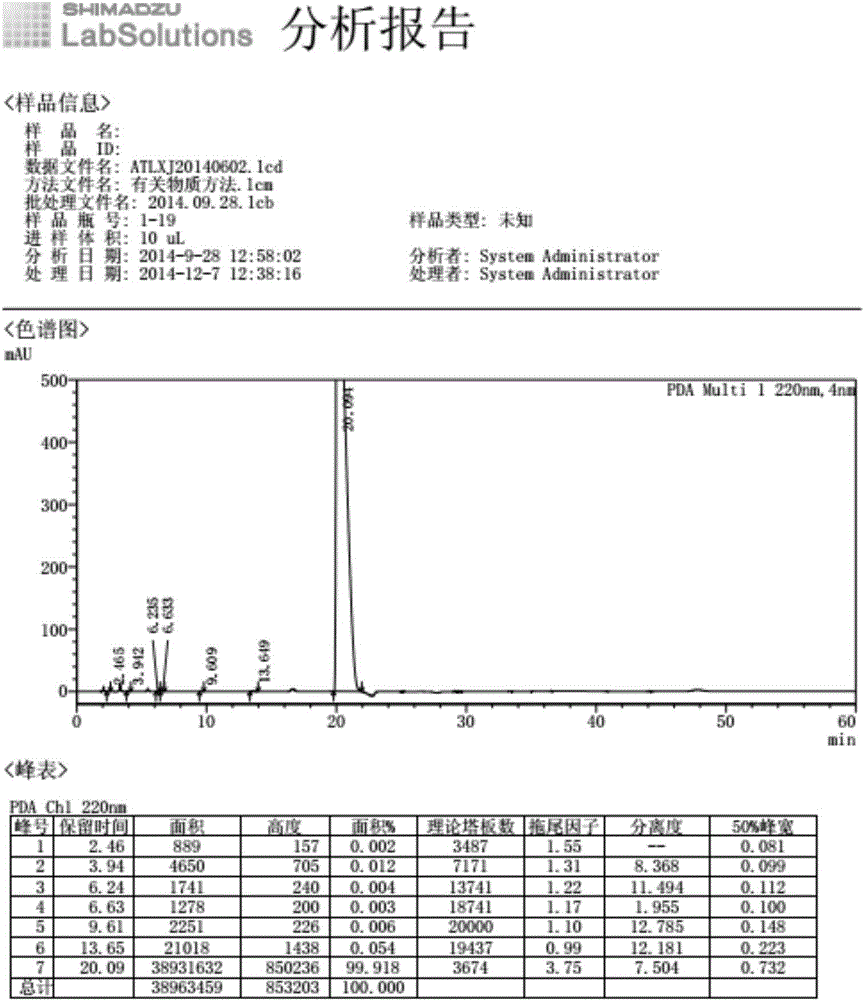
[0038] At room temperature, dissolve 770g potassium carbonate (6mol) in 1200ml purified water, add 4.8g potassium iodide (0.03mol), add 95g allantoin (0.6mol), stir for 30min, add potassium persulfate 33g (0.12mol) in batches, Stir at 40°C until the reaction of the raw materials is complete, filter out the insoluble matter, add glacial acetic acid to the filtrate to adjust the pH to 6, precipitate a solid, and dry to obtain 338g of the product with a purity of 99.8% and a maximum of 0.02%.
Embodiment 3
[0039] The preparation of embodiment 3 oteracil potassium
[0040] At room temperature, dissolve 336g potassium hydroxide (6mol) in 1200ml purified water, add 95g allantoin (0.6mol), add 4.8g potassium iodide (0.03mol), stir for 30min, add potassium persulfate 33g (0.12mol) in batches , Stir at 30°C until the reaction of the raw materials is complete, filter out the insoluble matter, adjust the pH of the filtrate to 6±0.5 with hydrochloric acid, precipitate a solid, and dry to obtain 306g of the product with a purity of 99.8% and a maximum of 0.042%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com