Monoclone antibody for anti HLA-G and hybrid tumour cell secreting same, cancer dignosis method, diagnosis reagent box and its application
A technology of hybridoma cell lines and monoclonal antibodies, which is used to detect abnormal expression of HLA-G in common malignant tumor lesions to diagnose cancer, immunohistochemical kits for diagnosing malignant tumors, hybridoma cell lines, and achieve monoclonal The effect of antibody stability, high sensitivity, and convenience for large-scale use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of monoclonal antibody of the present invention
[0038] The basis of the present invention is to use the natural HLA-G protein purified from placental tissue aborted within three months of pregnancy, which contains all HLA-G transcription isomers and glycoprotein isomers, to prepare anti-HLA-G Monoclonal antibodies. (See Ishitani et al., Proc.Natl.Acad.Sci.U.S.A.85:3947,1992; Kirszenbaum et al., Acad.Sci.U.S.A.91:4209,1994; Fujii et al., J.Immunol.153:5516,1994; McMaster et al., J. Immunol. 160:5922, 1998; Blastchitze et al., Early Pregnancy 5:67I 2001.)
[0039] 1. Purification of HLA-G protein
[0040] ●Immediately collect the postoperative embryonic tissues of abortion patients within three months, and freeze them at -80°C for future use.
[0041] ●When purifying HLA-G protein, thaw frozen tissue on ice and cut into small pieces. The tissue was suspended with 50 mM Tris-HCl buffer (pH 7.4), and homogenized with a Brinkmann Polytron homogenizer at 4...
Embodiment 2
[0055] Characteristics of the monoclonal antibody of the present invention
[0056] The following experiments were carried out with the monoclonal antibody prepared above to prove that:
[0057] 1. As determined by the mouse antibody typing kit (Amersh), the subtype of the monoclonal antibody produced by the cell line is IgG3.
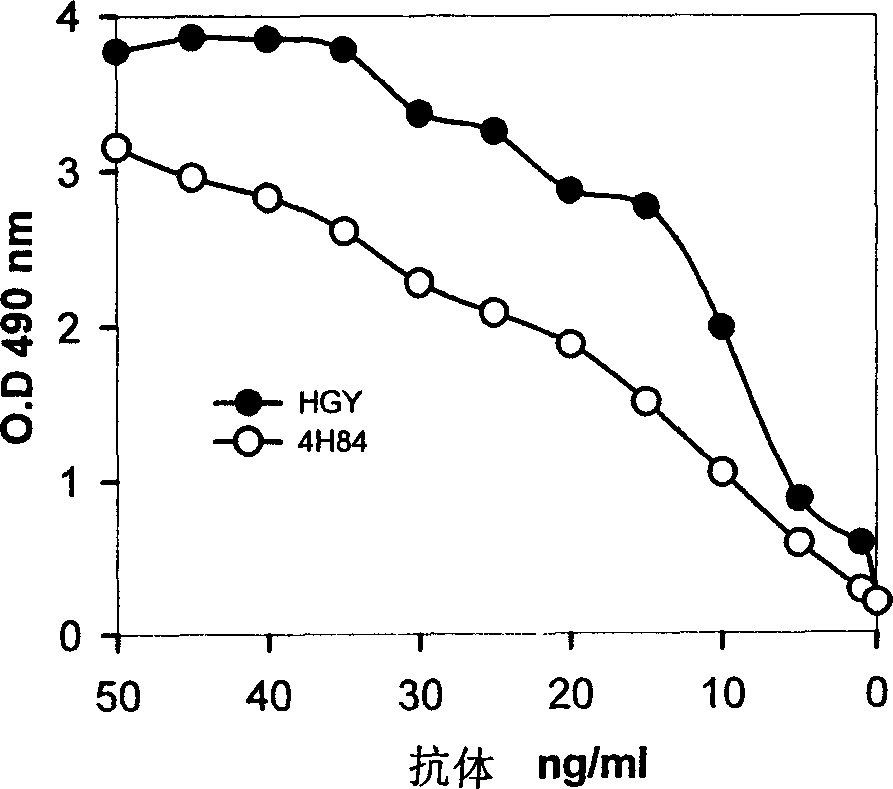
[0058] 2. It is highly specific for HLA-G
[0059] Antibody capture ELISA assays show that the antibodies of the present invention have no cross-reactivity with other types of HLA group I antigens, (see Gosling and Basso, Immunoassay, Butterworth-Hainemann, London,) confirming that the antibodies of the present invention have a high degree of HLA-G specificity. (See Table 1)
[0060] compound
HLA-G
HLA-A2
HLA-B4
HLA-C
mixed white blood cells
100
0.01
0.01
0.001
0.005
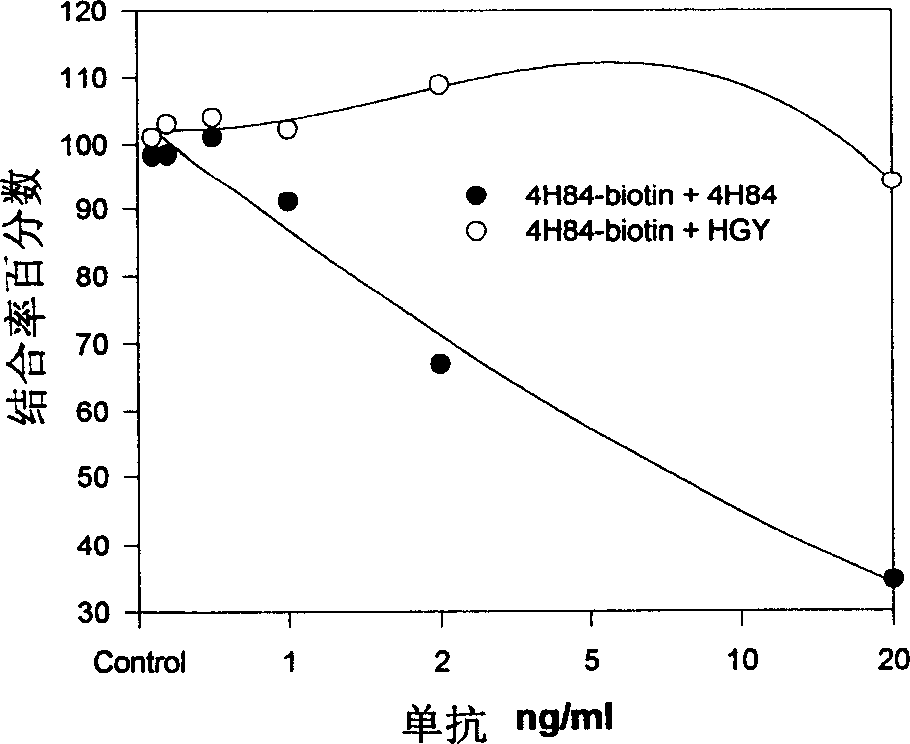
[0061] 3. Compared with the existing anti-HLA-G monoclonal antibody, it has higher antigen a...
Embodiment 3
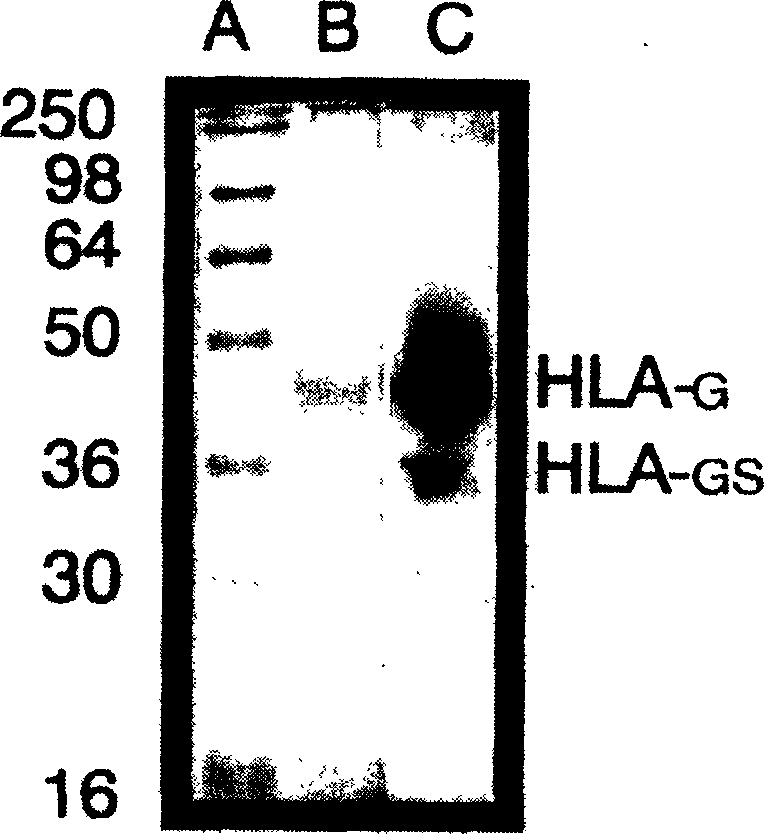
[0070] Western blot detection of HLA-G in cell lines and breast cancer samples
[0071] preface
[0072] Downregulation of classical human leukocyte antigen (HLA) family I expression is a common phenomenon in tumor biology. This change in the expression of the classical HLAI family may enable tumor cells to escape the immune surveillance of host toxic lymphocytes. Because NK cells are capable of hydrolyzing classical HLA group I deficient cells, the above changes can expose tumor cells with downregulated human leukocyte antigen (HLA) group I expression to NK cell attack. Garrido et al., Immunology Today 18:89, 1997. It has been found that, as a non-classical group I antigen, one of the immunological functions of HLA-G is to inhibit the activity of NK cells. Therefore, if tumor cells express HLA-G, they can be protected from NK destruction. To test this hypothesis, we investigated five human cancer cell lines and primary breast cancer tissues by immunoblotting with a spec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com