Preparation of multi-epitope TK-1 antibody, and application of multi-epitope TK-1 antibody in evaluating treatment effect on tumor patient
A multi-epitope, antibody technology, applied in the direction of anti-enzyme immunoglobulin, egg-derived immunoglobulin, measuring device, etc., can solve the problems such as the need to improve the sensitivity, not suitable for physical examination screening, etc., to prolong survival and quality of life , easy commercial transportation, high sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Selection of specific polyepitope antigens
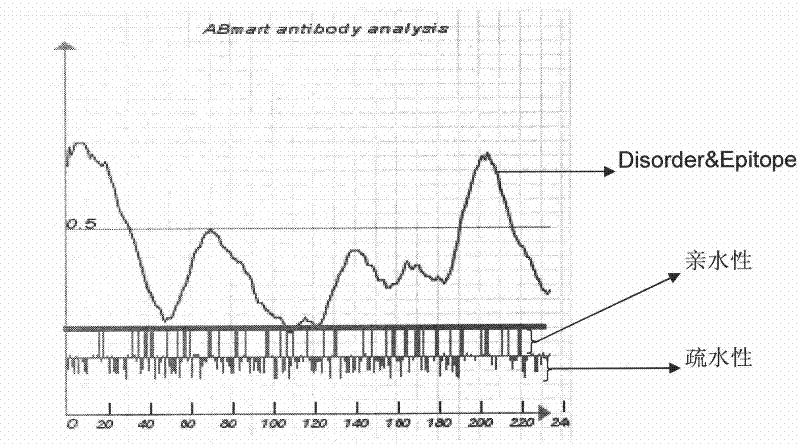
[0046] Such as figure 1 As shown, the software predicts that TK1 has no signal peptide and no transmembrane structure. The diagram shows the distribution of 234 amino acids in the single-chain TK1 protein, indicating that the distribution of amino acids is relatively uniform. The map provides options: 1) From the perspective of antigenicity, it is better to select the peptide at the place where the blue curve peak is high; 2) According to the distribution of hydrophilic amino acids, select the hydrophilic region; 3) Select the negative value Aromatic amino acids with strong hydrophobicity.
example 2
[0047] Example 2: Select the antigen of high sensitivity, high specificity multi-site combined anti-human TK1-IgY antibody
[0048] Selection of antigens for the preparation of highly sensitive and highly specific multi-site combined anti-human TK1-IgY antibodies: Since the partial sequence of human cervical cancer TK1 has the same epitope as other proteins, the obtained antibodies may be different from other non-specific proteins There is cross-reaction and purification is difficult. According to the published and known TK1 sequence (protein library), select the special peptide exposed on the surface of the protein. According to the specificity of the polypeptide predicted by the software, the polypeptide fragments are listed in the following table. Antigen fragments with strong TK1 specificity were screened, and TK1 N-terminal 23 peptide, C-terminal 20 peptide and C-terminal 28 peptide were designed as haptens.
[0049]
[0050]
Embodiment 3
[0051] Example 3: Screening for specific polyepitope antigens
[0052] Selection of specific multi-epitope antigen: according to the well-known spatial structure of TK1, select the antigenic determinant exposed on the surface of TK1 protein, screen the antigenic fragment with strong immunity of TK1 according to Example 2, screen the N-terminal 23 peptide of TK1, and the C-terminal The 20 peptide and the C-terminal 28 peptide are antigen fragments with strong immunity. The amino acid sequences of the three segments are as follows (see sequence listing): 1) N-terminal 23 peptide (3-25): CINLPTVLPGSPSKTRGQIQVIL, 2) C-terminal 20 peptide (206 -225): CPVPGKPGEAVAARKLFAPQ, 3) C-terminal 28 peptide (198-225): AGPDNKENCPVPGKPGEAVAARKLFAPQ.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com