Hyaluronic acid-gelatin composite hydrogel carrying PLGA microspheres and preparation method thereof
A technology of composite hydrogel and hyaluronic acid, which is applied in the fields of pharmaceutical formulation, medical science, drug delivery, etc., can solve the problems of patients with harmful side effects, and achieve excellent swelling properties, slow release of antibacterial properties of drugs, and low mechanical properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
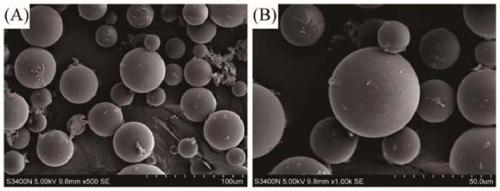
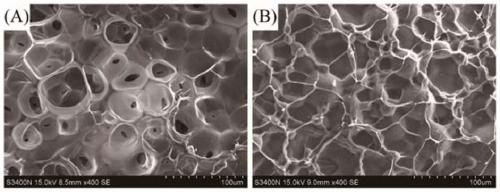
[0036] A hyaluronic acid-gelatin composite hydrogel loaded with PLGA microspheres, the hydrogel comprises the following components in mass percent: 12.5% aminoethyl methacrylate hyaluronic acid, 10% methacrylated gelatin, PLGA@GS composite microspheres 0.5%, photoinitiator 0.1%, and water as the balance.
[0037] The preparation method of the hydrogel in this example is: 0.25g HA-AEMA (12.5wt.%), 0.2g GelMA (10wt.%), 10mg PLGA@GS microspheres (0.5wt.%) and 2mg LAP photoinitiated HA-AEMA / GelMA / PLGA@GS hydrogel was obtained by dissolving GelMA (0.1wt.%) in 2mL deionized water and irradiating with ultraviolet light for 20min.
[0038] Among them, the preparation method of aminoethyl methacrylate hyaluronic acid is: weigh 1g hyaluronic acid and dissolve it in 100mL distilled water, after the hyaluronic acid is completely dissolved, add 0.25g EDC, 0.125g NHS, 0.125g AEMA, and then React at room temperature for 24 hours, dialyze with a cellulose dialysis bag with a molecular weig...
Embodiment 2
[0042] A hyaluronic acid-gelatin composite hydrogel loaded with PLGA microspheres, the hydrogel comprises the following components in mass percent: 1% aminoethyl methacrylate hyaluronic acid, 20% methacrylated gelatin, 0.01% of PLGA@GS composite microspheres, 0.01% of photoinitiator, and the balance is water.
[0043] The preparation method of the hydrogel in this example is as follows: 0.01g HA-AEMA (1wt.%), 0.4g GelMA (20wt.%), 0.2mg PLGA@GS microspheres (0.01wt.%) and 0.2mg LAP light The initiator (0.01wt.%) was dissolved in 2 mL of deionized water, and irradiated with ultraviolet light for 20 min to obtain the HA-AEMA / GelMA hydrogel.
[0044] The preparation method of aminoethyl methacrylate hyaluronic acid, methacrylated gelatin, and PLGA@GS composite microspheres is the same as in Example 1.
Embodiment 3
[0046] A hyaluronic acid-gelatin composite hydrogel loaded with PLGA microspheres, the hydrogel comprises the following components in mass percent: 20% aminoethyl methacrylate hyaluronic acid, 1% methacrylated gelatin, PLGA@GS composite microspheres 1%, photoinitiator 0.5%, and water as the balance.
[0047] The preparation method of the hydrogel in this embodiment is: 0.2g HA-AEMA (20wt.%), 0.02g GelMA (1wt.%), 20mg PLGA@GS microspheres (1wt.%) and 10mg LAP photoinitiator ( 0.5 wt.%) was dissolved in 2 mL of deionized water, and irradiated with ultraviolet light for 20 min to obtain HA-AEMA / GelMA hydrogel.
[0048] The preparation method of aminoethyl methacrylate hyaluronic acid, methacrylated gelatin, and PLGA@GS composite microspheres is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com