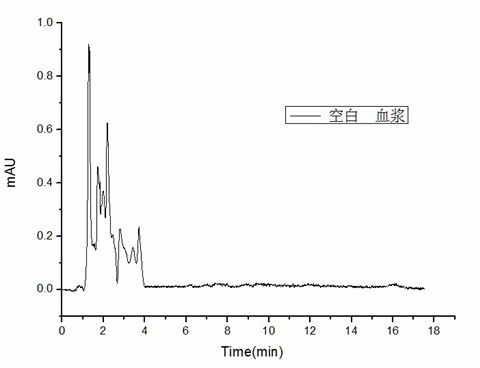
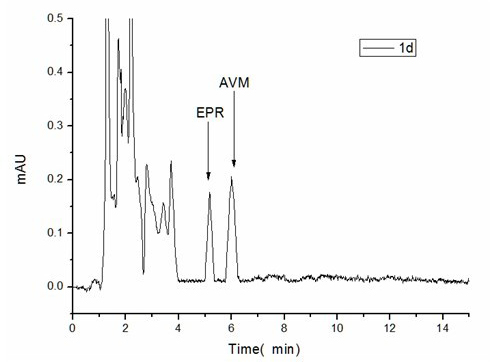
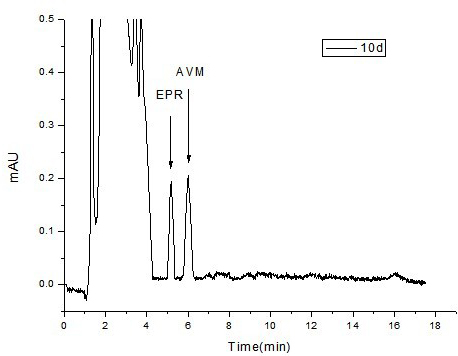
Acetyl amino abamectin sustained-release microsphere and preparation method as well as sustained-release microsphere injection
A technology of acetamido abamectin and slow-release microspheres, which can be applied in pharmaceutical formulations, medical preparations containing active ingredients, and devices for making medicines into special physical or taking forms, etc. It can improve the bioavailability, reduce the cost of medication, and reduce the number of times of administration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: The acetamidoabamectin sustained-release microspheres were prepared by the following method.
[0025] 1. Dissolve 250mg of acetaminobamectin and 1g of PLGA (50 / 50 DLG 4E Mw=57000) in 10ml of dichloromethane to fully dissolve to form an oil phase.
[0026] 2. Prepare 0.5L aqueous solution of polyvinyl alcohol with a concentration of 1wt% (the degree of alcoholysis is 88%, the degree of polymerization is 1725, and the weight average molecular weight is 30,000), so as to ensure that the polyvinyl alcohol is completely dissolved in water to form an aqueous phase; The injection pump injects the oil phase into the vortex-shaped PVA water phase dispersion medium at a constant speed through a conical nozzle with an inner diameter of 0.12 mm. During the injection process, the oil phase stream at the outlet of the nozzle obtains a constant shear force, forming an oil-in-water emulsion. Stir at room temperature for 6 hours to solidify the microspheres.
[0027] 3. T...
Embodiment 2
[0030] Embodiment 2: The acetamidoabamectin sustained-release microspheres were prepared by the following method.
[0031] 1. Dissolve 250mg of acetaminobamectin and 1g of PLGA (50 / 50 DLG 9E Mw=155000) in 20ml of dichloromethane to fully dissolve to form an oil phase.
[0032] 2. Prepare 0.7L of polyvinyl alcohol (PVA) aqueous solution with a concentration of 1wt% (alcoholysis degree of 90%, polymerization degree of 1750, weight average molecular weight of 50,000) to make the water phase; use a syringe pump to pass the oil phase through 0.12 A conical nozzle with an inner diameter of mm is injected into the vortex-shaped PVA water-phase dispersion medium at a constant speed. During the injection process, the shear force obtained by the fine flow of the oil phase at the outlet of the nozzle is constant, forming an oil-in-water emulsion. Stir at room temperature for 18 hours to make the microspheres solidify.
[0033]3. The solidified microspheres were centrifuged and washed th...
Embodiment 3
[0036] Example 3: The acetamidoabamectin sustained-release microspheres were prepared by the following method.
[0037] 1. Dissolve 467mg of acetamidobamectin and 1g of PLGA (65 / 35 DLG 5E IV=0.5) in 15ml of dichloromethane to fully dissolve to form an oil phase.
[0038] 2. Prepare 1.0L of polyvinyl alcohol (PVA) aqueous solution with a concentration of 3wt% (alcoholysis degree of 87%, polymerization degree of 1700, weight average molecular weight of 20,000) to make the water phase; use a syringe pump to pass the oil phase through 0.20 The conical nozzle with an inner diameter of mm injects the vortex-shaped PVA water phase dispersion medium at a constant speed. During the injection process, the oil phase stream at the outlet of the nozzle obtains a constant shear force to form an oil-in-water emulsion. Stir at room temperature for 12 hours to make the micro The ball solidifies.
[0039] 3. The solidified microspheres were centrifuged and washed three times with water, collec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com