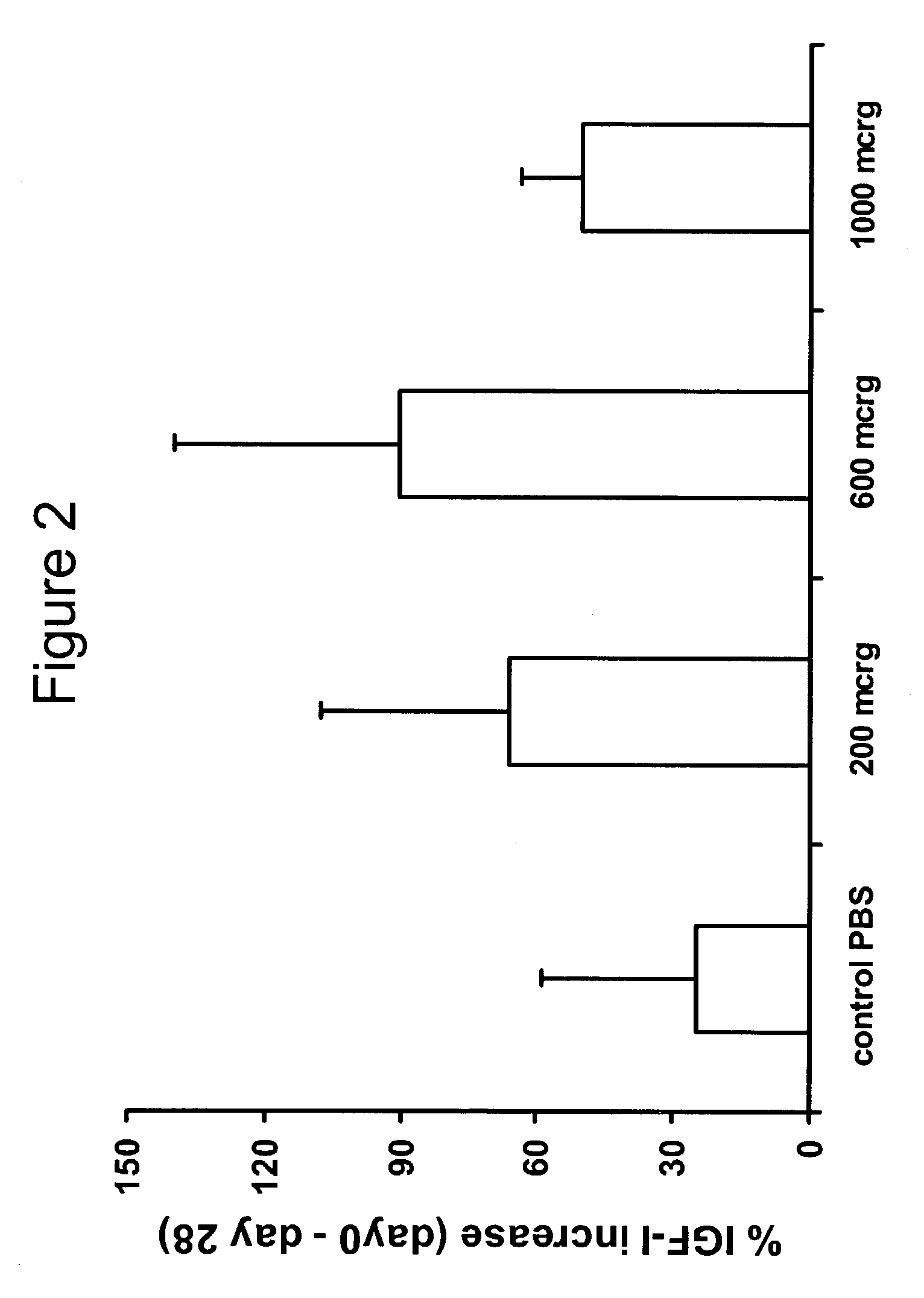
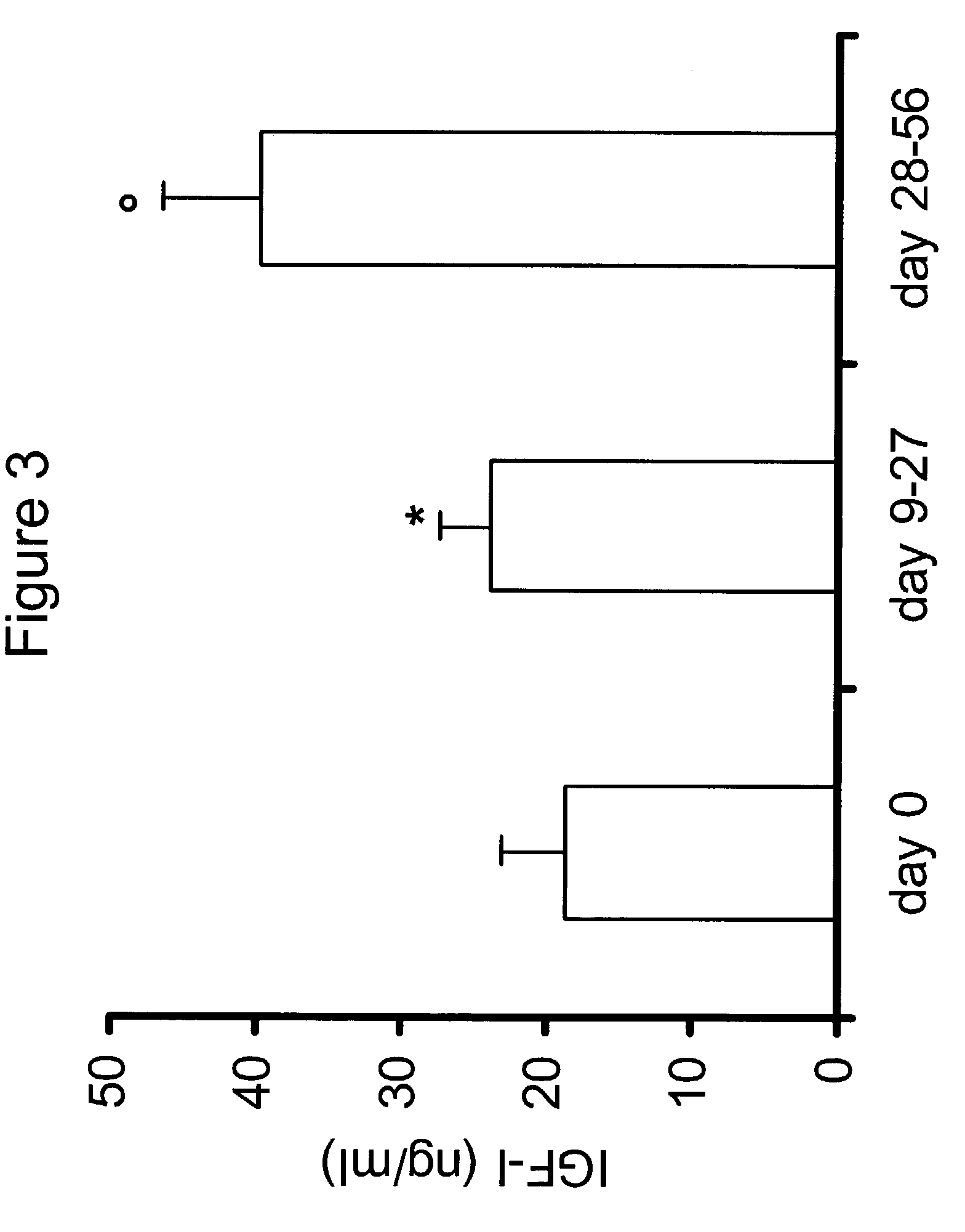
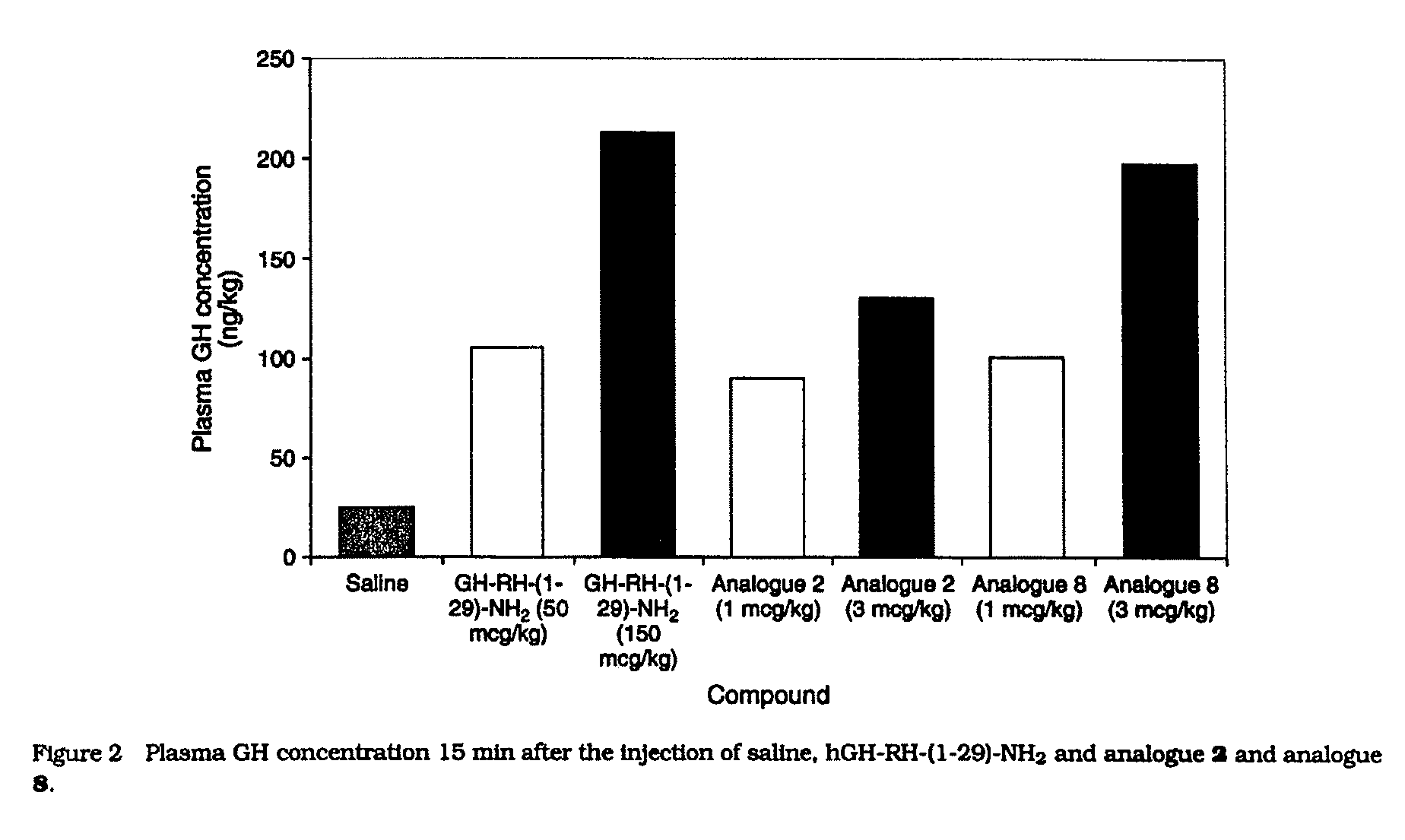
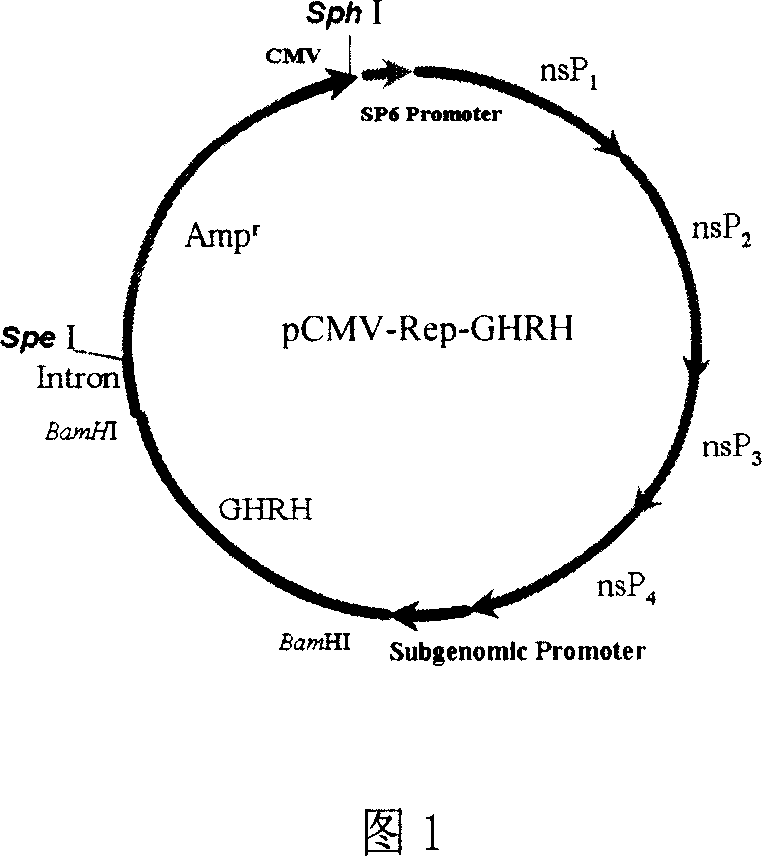
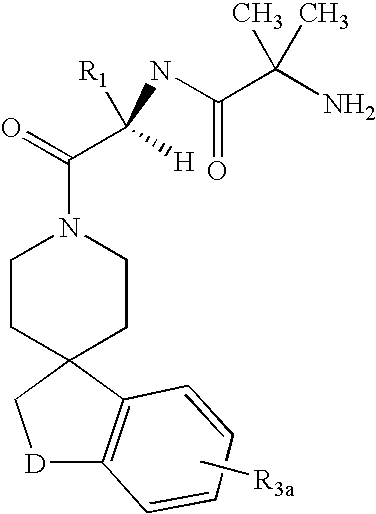
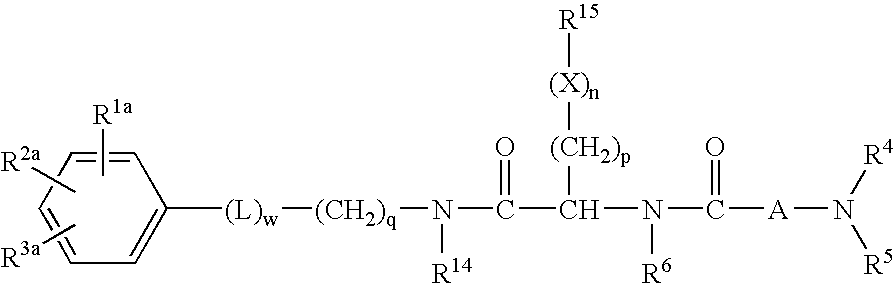
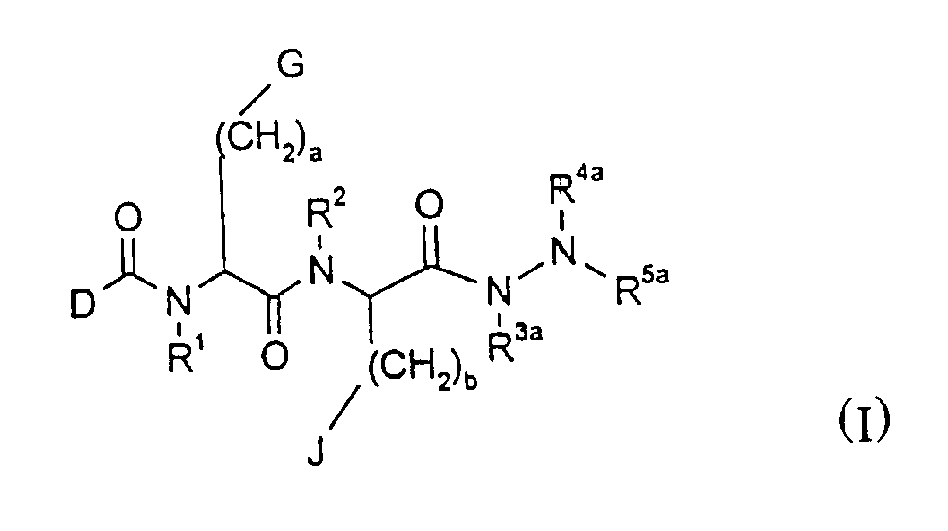
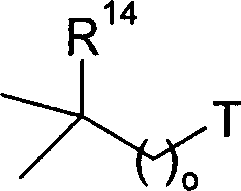
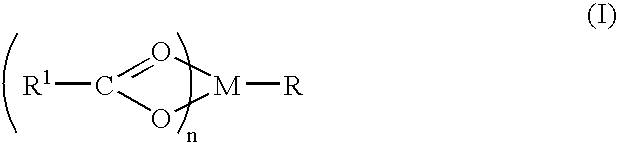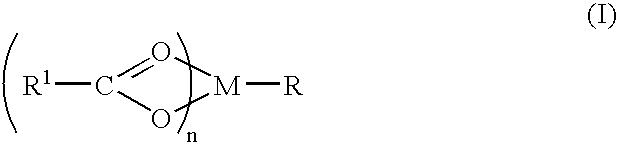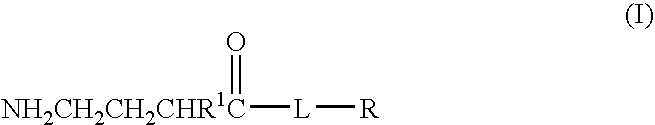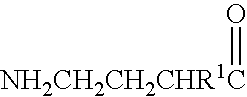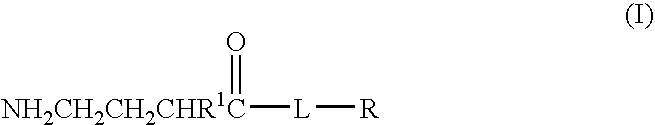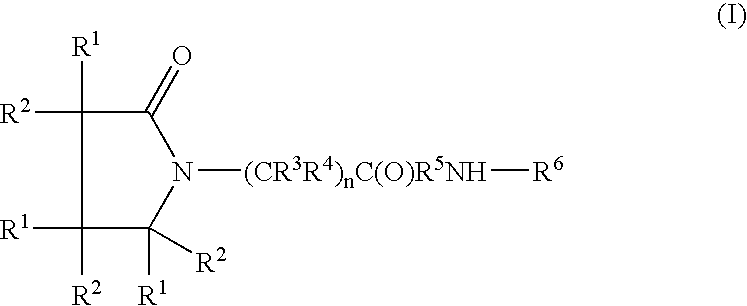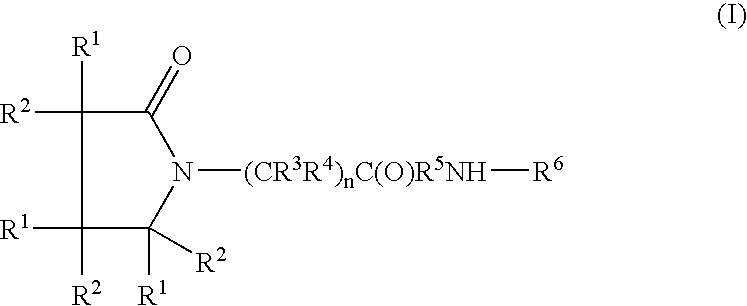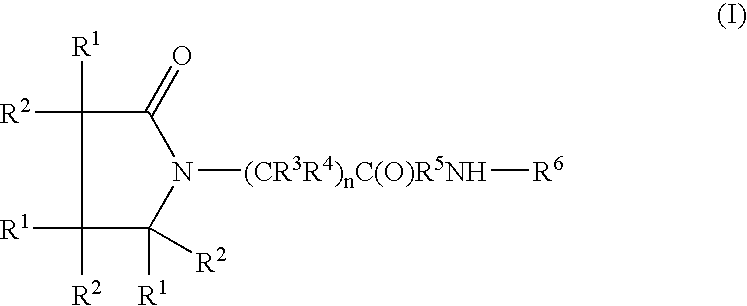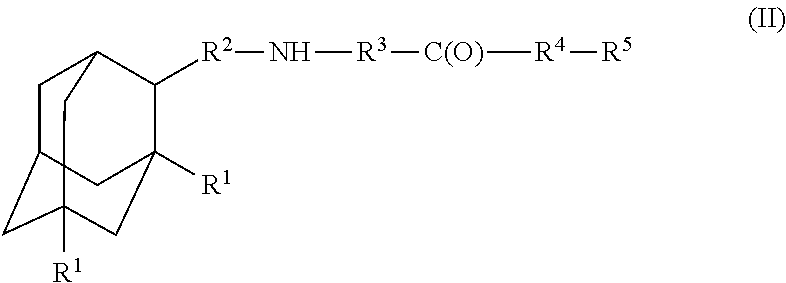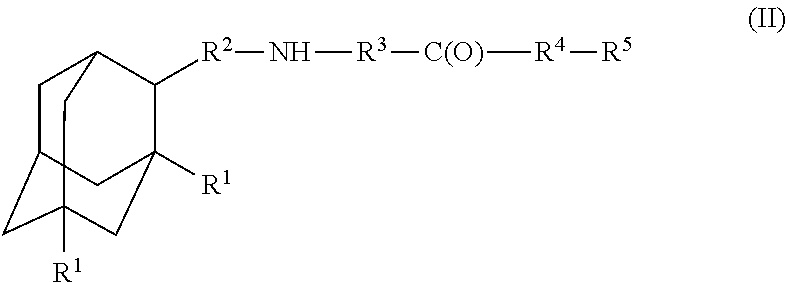Patents
Literature
49 results about "Growth hormone–releasing hormone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Growth hormone–releasing hormone (GHRH), also known as somatocrinin or by several other names in its endogenous forms and as somatorelin (INN) in its pharmaceutical form, is a releasing hormone of growth hormone (GH). It is a 44-amino acid peptide hormone produced in the arcuate nucleus of the hypothalamus.
Compound and method of treating neurogenic conditions using non-steroidal anti-inflammatory drug complexes
A complex is provided for the treatment of neurogenic conditions having the formula: where R1 is M is a metal ion Ca(II), Mg(II), Cu(II) or Ni(II); n is an integer 1 or 2; R is BBB peptide, transferrin, membrane transporter peptide, TAT peptide, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, L-lactate, L-leucine, L-tryptophan, and L-glutamate; and R is coupled to M through a carboxylate moiety. Magnesium (II) represents the preferred metal ion as magnesium is known to have neuroprotective effects. The metal ion is in part chelated by a non-steroidal anti-inflammatory drug that does not inhibit platelet activity and includes salicylate and ibuprofenate. The complex also includes a ligand operative in transport across the blood brain barrier. A process for making an inventive complex includes the stoichiometric addition of ligands containing carboxylate groups to a solution of the metal ion. In instances where the metal ion is magnesium (II), a stoichiometric ratio of 1:1:1 is found between the non-steroidal anti-inflammatory ligand:magnesium (II):transporter ligand.
Owner:MILLER LANDON C G
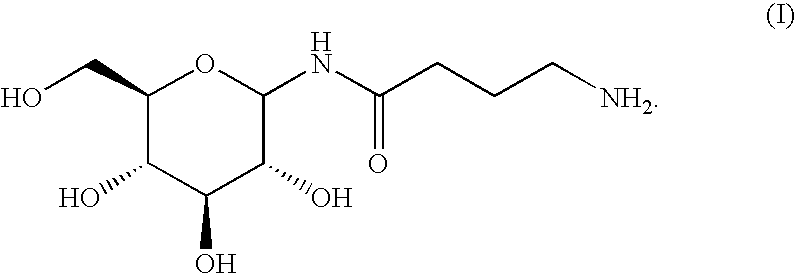
Aminobutyramide conjugate and a pharmaceutical composition for treatment of neuronal disorders
ActiveUS20060058219A1Improve efficiencyEliminate side effectsBiocidePeptide/protein ingredientsTryptophanSaccharin
A compound is provided that has the formula NH2CH2CH2CH2C(O)N—R (I) where R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidetransferrin, glucosylamnine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
Compounds with growth hormone releasing properties
InactiveUS6274584B1Promote growthIncrease ratingsPowder deliveryBiocideMedical disorderBioavailability
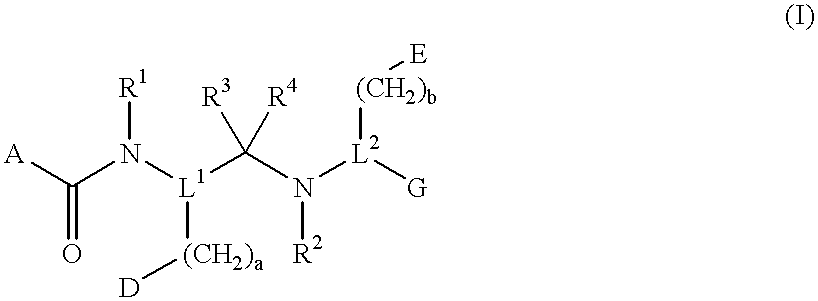
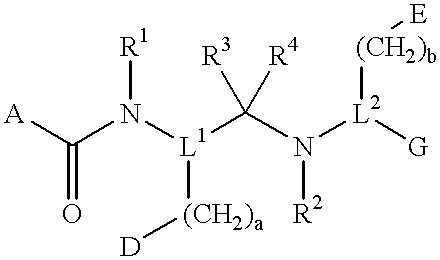
Novel peptide derivatives, compositions containing them, and their use for treating medical disorders resulting from a deficiency in growth hormone are disclosed. The peptides have the formula (1):wherein a, b, A, R1, L1, D, R3, R4, R2, L2, E and G are as defined in the specification. These peptides exhibit improved resistance to proteolytic degradation, and hence, improved bioavailability.
Owner:HELSINN THERAPEUTICS (US) INC
VPAC1 selective antagonists and their pharmacological methods of use
InactiveUS20050203009A1High activityHigh expressionPeptide/protein ingredientsAntibody mimetics/scaffoldsVpac1 receptorGrowth hormone–releasing hormone
The disclosed invention relates to selective VPAC1 antagonists, related formulations, dosages and methods of use. The selective VPAC1 antagonists of the invention comprise a vasoactive intestinal peptide component and a growth hormone releasing hormone component capable of selectively binding to and antagonizing the VPAC1 receptor at significantly lower concentrations than those concentrations at which it binds to and antagonizes the VPAC2 receptor.
Owner:BAYER PHARMA CORP
Baclofen conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound is provided that has the formula NH2CH2CH2CHR1C(O)N—R (I) where R1 is p-chlorophenyl, R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptide transferrin, glucosylamine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
Aminobutyramide conjugate and a pharmaceutical composition for treatment of neuronal disorders
ActiveUS7074775B2Improve efficiencyEliminate side effectsBiocideDipeptide ingredientsTryptophanSecretin
A compound is provided that has the formulaNH2CH2CH2CH2C(O)N—R (I)where R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidetransferrin, glucosylamnine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
Baclofen conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound is provided that has the formulaNH2CH2CH2CHR1C(O)N—R (I)where R1 is p-chlorophenyl, R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptide transferrin, glucosylamine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
Treatment of HIV-associated dysmorphia/dysmetabolic syndrome (HADDS) with or without lipodystrophy
Owner:MERCK SERONO SA
Piracetam and piracetam analog conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound of the formulawhere R1 is H, C1–C4 alkyl and OH; R2 in is H, C1–C4 alkyl and OH; R3 is H and C1–C4 alkyl; R4 is H and C1–C4 alkyl; n is an integer between 0 and 2 inclusive; R5 is a nullity, NHR7C(O)—, C6H4—, C6H4—O—; R7 is C2–C6 alkyl; and R6 is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine, blood brain barrier (BBB) peptide, membrane translocating peptide, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, transferrin, glucosylamine, amino saccharin, saccharin ester, lactylamine, leucine, tryptophan, amino glutamate and amino cholines.
Owner:MILLER LANDON C G
Low molecular weight peptidomimetic growth hormone secretagogues
InactiveUS20030139348A1Facilitated releaseIncrease secretionTetrapeptide ingredientsTripeptide ingredientsMammalGrowth promoting
Owner:GENENTECH INC
Piracetam and piracetam analog conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound of the formula where R1 is H, C1-C4 alkyl and OH; R2 in is H, C1-C4 alkyl and OH; R3 is H and C1-C4 alkyl; R4 is H and C1-C4 alkyl; n is an integer between 0 and 2 inclusive; R5 is a nullity, NHR7C(O)—, C6H4—, C6H4—O—; R7 is C2-C6 alkyl; and R6 is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine, blood brain barrier (BBB) peptide, membrane translocating peptide, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, transferrin, glucosylamine, amino saccharin, saccharin ester, lactylamine, leucine, tryptophan, amino glutamate and amino cholines.
Owner:MILLER LANDON C G
Treating anemia in subjects by administration of plasmids encoding growth hormone releasing hormone
InactiveUS7241744B2Quality improvementBiocidePeptide/protein ingredientsAbnormal tissue growthWilms' tumor
The present invention pertains to compositions and methods for plasmid-mediated supplementation. The compositions and method are useful for retarding the growth of the tumor, and retarding cachexia, wasting, anemia and other effects that are commonly associated in cancer bearing animals. Overall, the embodiments of the invention can be accomplished by delivering an effective amount of a nucleic acid expression construct that encodes a GHRH or functional biological equivalent thereof into a tissue of an animal and allowing expression of the encoded. gene in the animal. For example, when such a nucleic acid sequence is delivered into the specific cells of the tissue specific constitutive expression is achieved.
Owner:INOVIO PHARMA +1
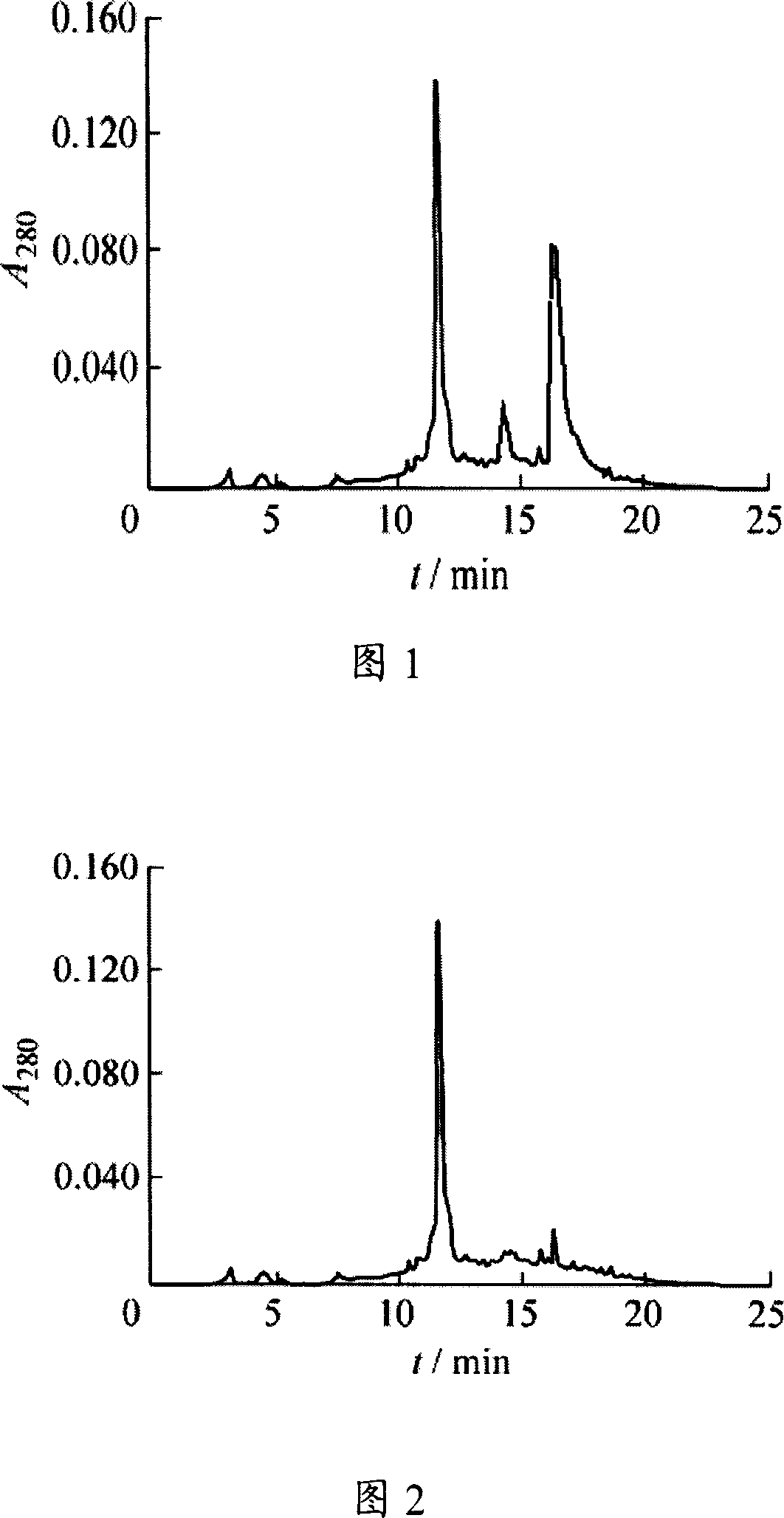
Mink growth hormone releasing hormone cDNA and application thereof
The invention discloses a full-length nucleotide sequence and a mature peptide nucleotide sequence of a mink growth hormone releasing hormone cDNA gene, and encoded protein amino acid sequences thereof. The mink growth hormone releasing hormone cDNA sequence is a complete open reading frame with 321 nucleotide sequences, and 106 amino acids are encoded. The mature peptide is encoded by 132 nucleotide sequences, and 44 amino acid residues are encoded. The mink growth hormone releasing hormone cDNA can be used for promoting the growths of economic animals such as minks.
Owner:CHINA AGRI UNIV
Low molecular weight peptidomimetic growth hormone secretagogues
InactiveUS20020111461A1Less effectDecreased insulin sensitivityTripeptide ingredientsImmunoglobulins against growth factorsMammalGrowth promoting
The present invention comprises growth hormone releasing peptides / peptidomimetics (GHRP) capable of causing release of growth hormone from the pituitary. Compositions containing the GHRP's of this invention are used to promote growth in mammals either alone or in combination with other growth promoting compounds, especially IGF-1. In a method of this invention GHRP's in combination with IGF-1 are used to treat Type II diabetes. An exemplary compound of this invention is provided below.
Owner:SOMERS TODD C +6
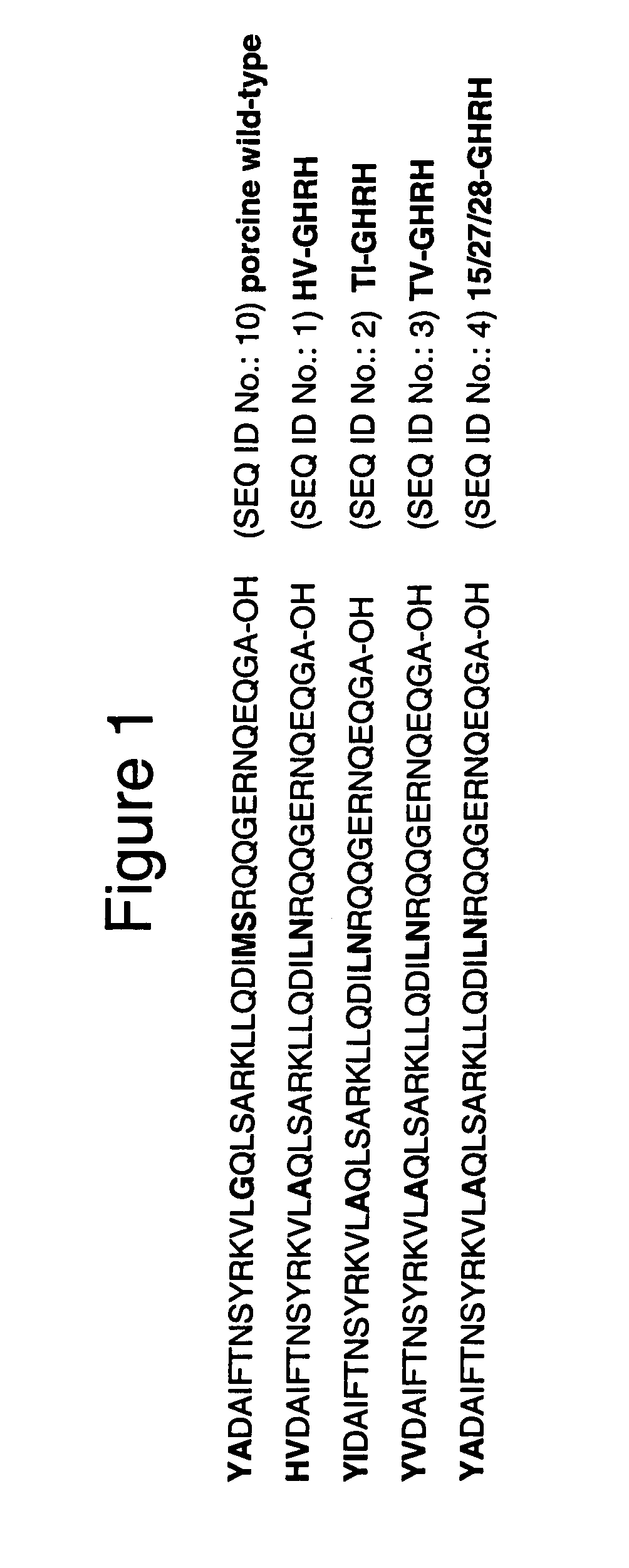
Analogs of human growth hormone-releasing hormone, their preparation and use
InactiveUS7928063B2Peptide/protein ingredientsPeptide preparation methodsSide chainEnzymatic degradation
Growth hormone-releasing hormone analogs having the amino acid sequence of the formula: Dat-Ala-Asp-Ala-Ile-Phe-Thr-Asn-Ser-Tyr10-R11-R12-VAL-Leu-Ala-Gln-Leu-Ser-Ala-R20-R21-Leu-Leu-Gln-Asp-Ile-Nle-Asp-R29-NH2 (I), wherein R11 is hArg, Gab or Gap; R12 is hArg, Orn, Gab or Gap; R20 is hArg, hArg, Gab or Gap; R21 is hArg, Orn, Gab or Gap; R29 is D-Arg, hArg, Gab or Gap; and their pharmaceutically acceptable salts. Said peptides are potent and selective stimulators of GH release and they are highly resistant to enzymatic degradation. Said peptides are prepared using the solid phase synthesis method, by introducing suitable derivatives of lysine, 2,4-diaminobutyric acid or 2,3-diaminopropionic acid at appropriate positions in the peptide chain attached to a polymeric support, deprotecting side-chain amino groups and reacting free amino groups with a guanidinating agent, removing all remaining t-butyloxycarbonyl protective groups, and cleaving the synthesized peptide from the support, followed by purification and optionally, converting the peptide into a pharmaceutically acceptable salt.
Owner:INSTITUT FARMACEUTYCZNY +1
Medicine for promoting pigling growth and improving pigling immunity and preparing method thereof
InactiveCN1927403APromote growthPromote growth and developmentGenetic material ingredientsPharmaceutical delivery mechanismMicrosphereGrowth hormone–releasing hormone
The invention relates to a drug used to accelerate the growth of pigling and improve its immunity, and relative production. Wherein, it is formed by PLGA microball that packing anterior pituitary growth hormone release hormone expression particle pCMV-Rep-GHRH; via ejecting said PLGA microball particles pCMV-Rep-GHRH into female pig, to express GHRH and improve the GH level, it can accelerate the growth of pigling and improve its immunity.
Owner:张永亮
Abstraction of pilose antler releasing somatomedin (DEER GHRF) and preparation method thereof
The present invention relates to process of extracting growth hormone release factor (GHRF) from pilose antler. The process includes the following steps: 1. crushing pilose antler and leaching to obtain leached liquid; 2. ultrafiltering the leached liquid and collecting the pilose antler extract of molecular weight 1-10 kDa; and 3. high efficiency liquid chromatography on the pilose antler extract and collecting component containing GHRF of pilose antler. The present invention also relates to the pilose antler extract with rich GHRF, and its application in medicine, food, cosmetics, feed, etc.
Owner:王利忠
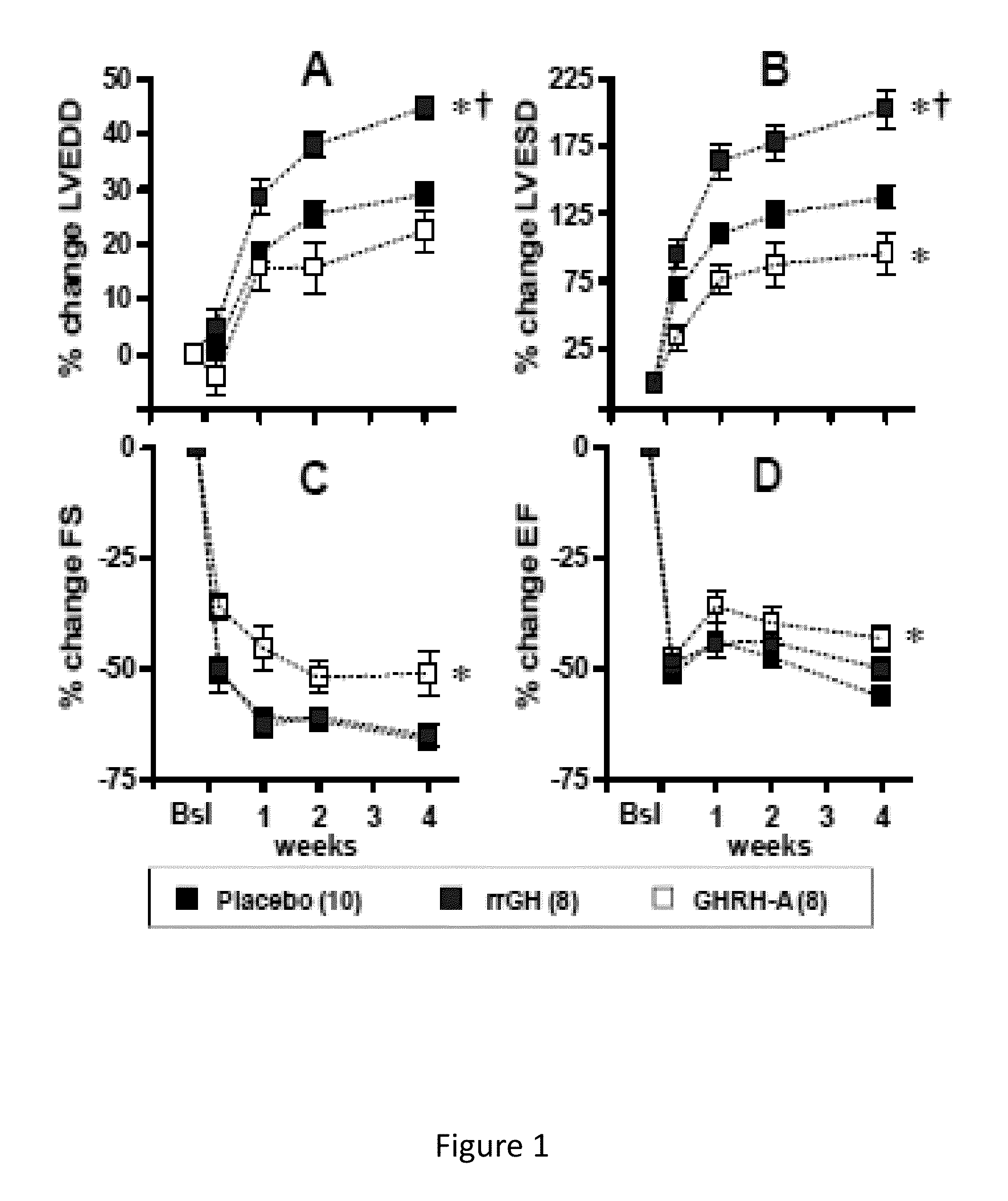
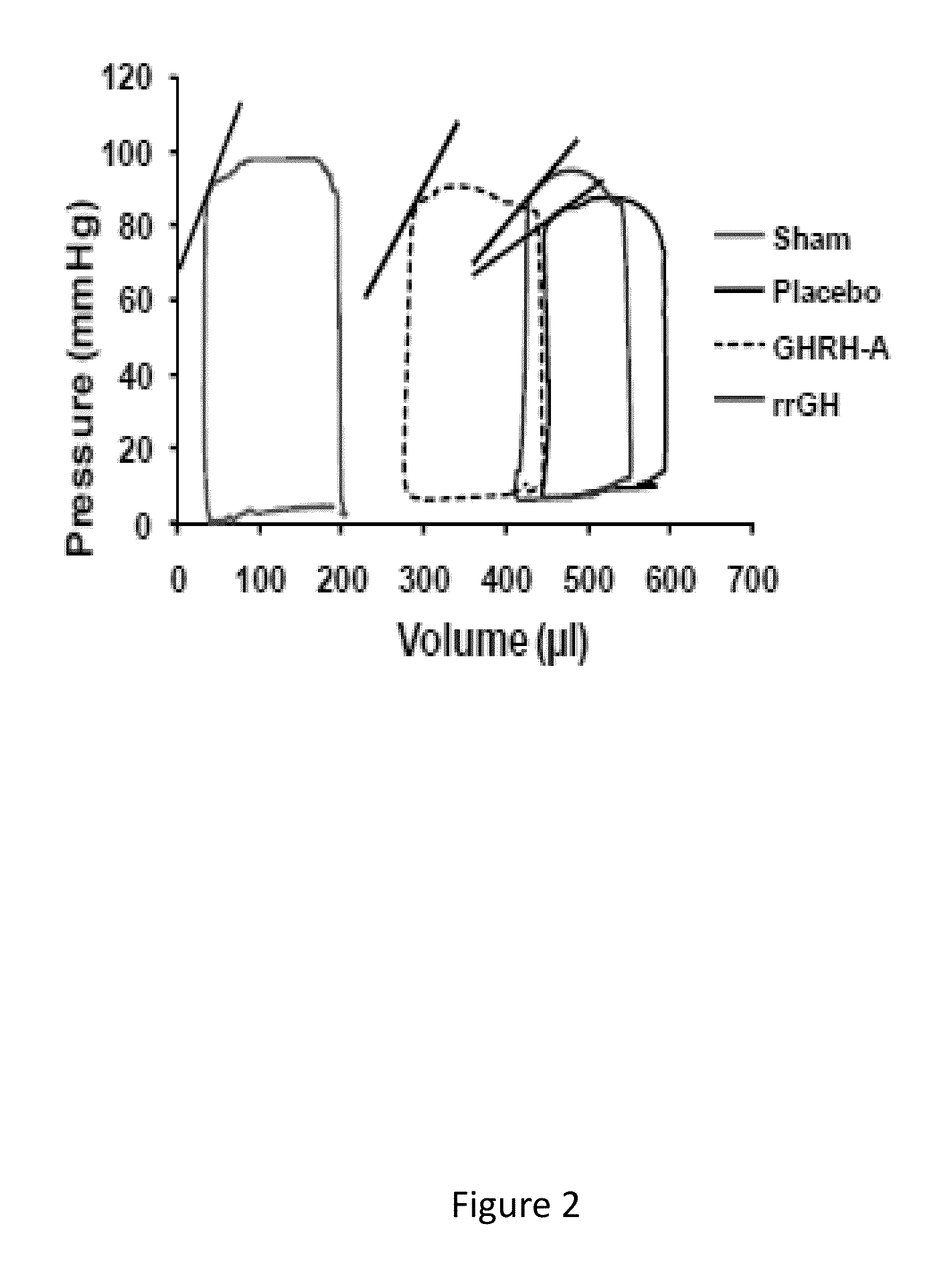
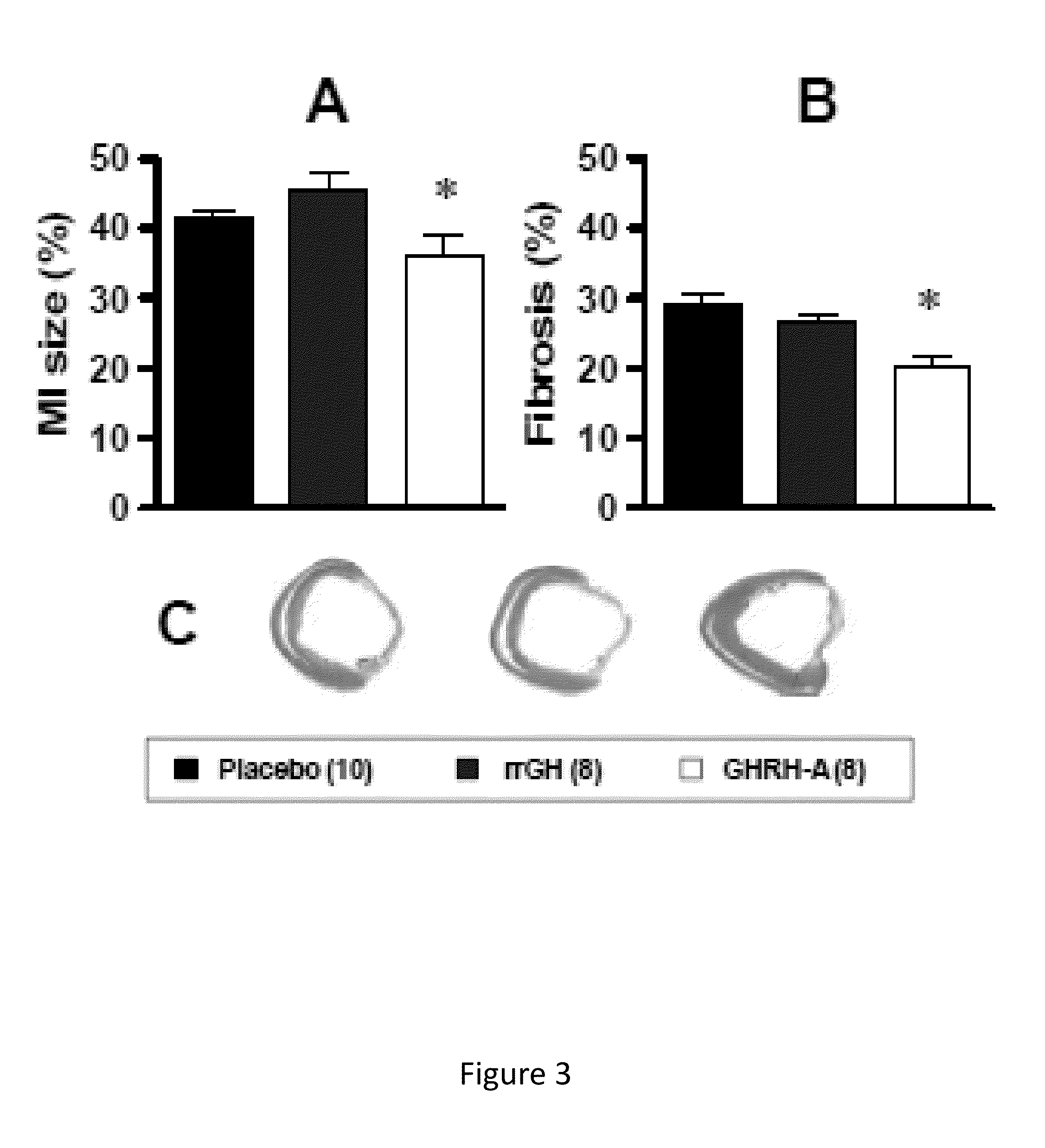
Cardioprotective effects of GHRH agonists
ActiveUS8507433B1Improved cardiac structureFunction increasePeptide/protein ingredientsAntinoxious agentsInsulin-like growth factorFunctional decline
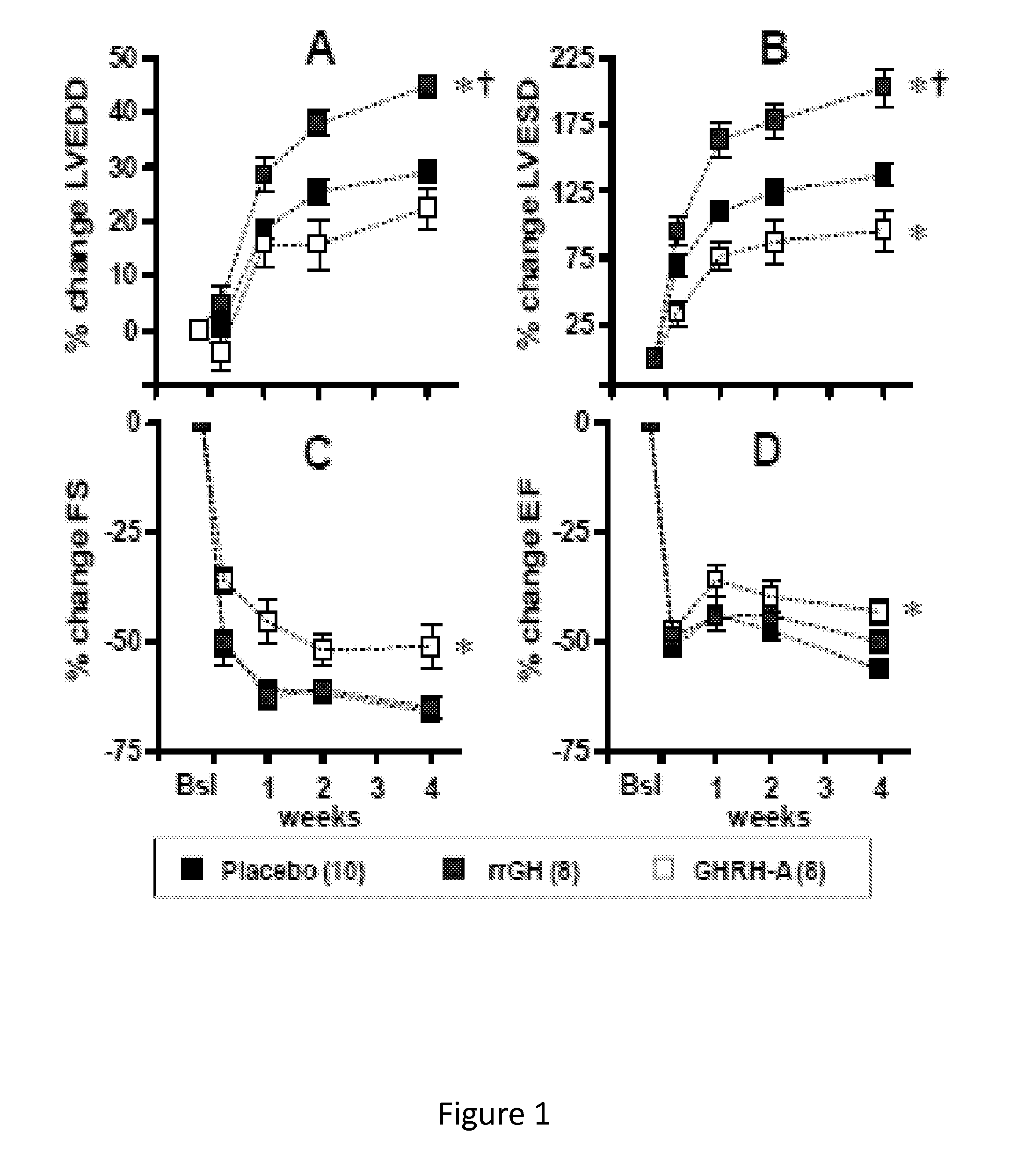
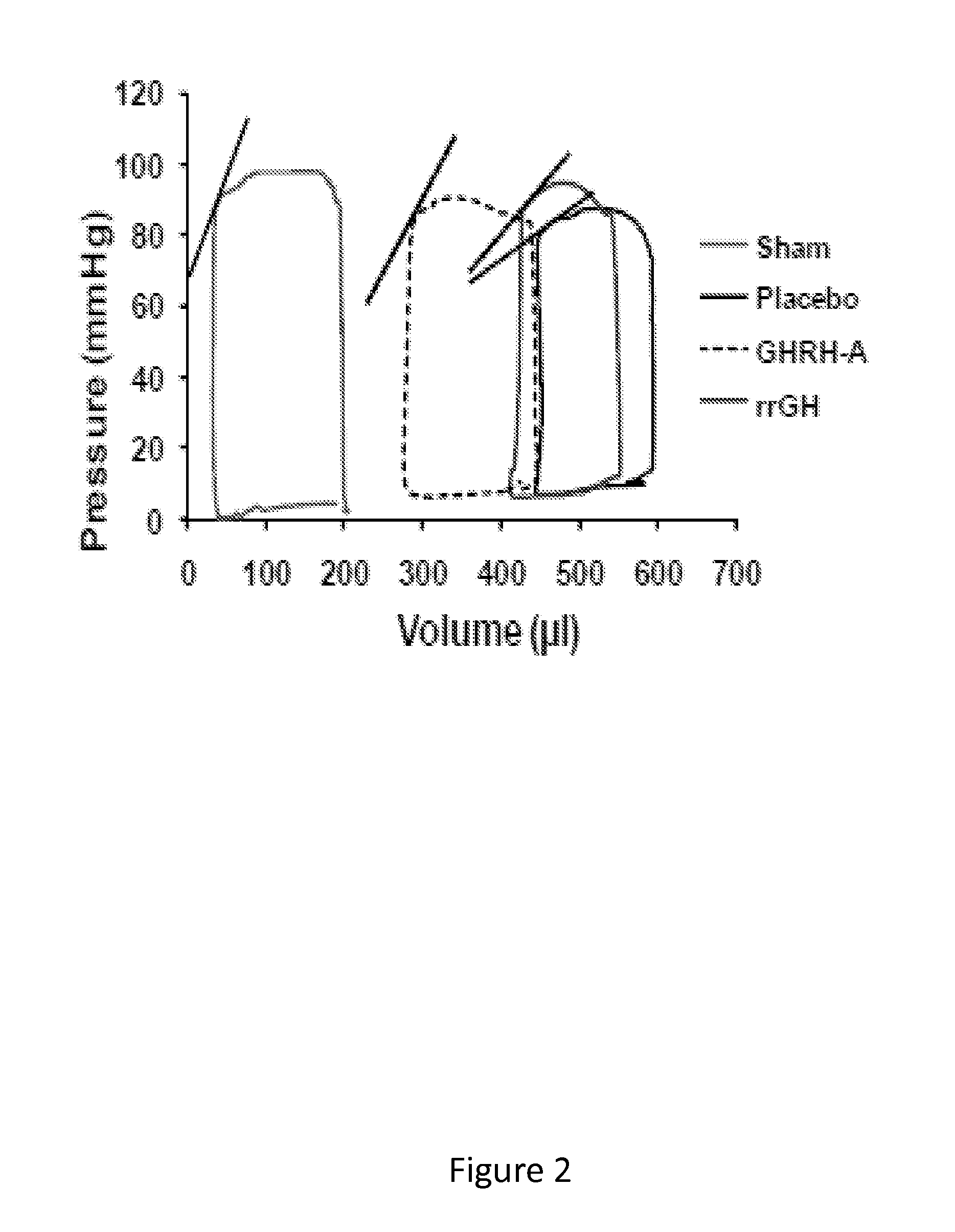
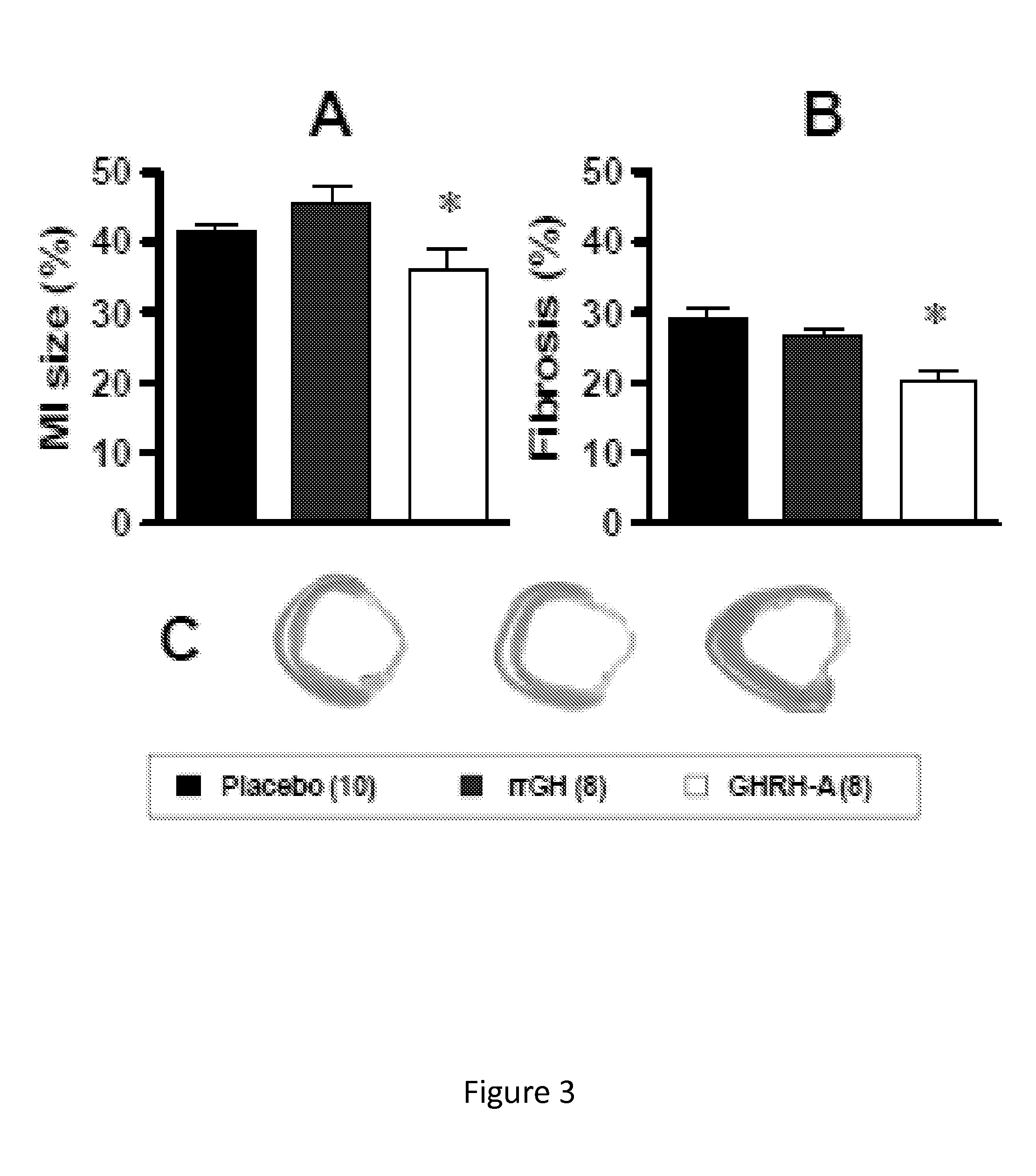
Whether the growth hormone (GH) / Insulin-like growth factor 1(IGF-I) axis exerts cardioprotective effects remains controversial; and the underlying mechanism(s) for such actions are unclear. Here we tested the hypothesis that growth-hormone releasing hormone (GHRH) directly activates cellular reparative mechanisms within the injured heart, in a GH / IGF-I independent fashion. Following experimental myocardial infarction (MI), rats were randomly assigned to receive, during a 4 week period, either placebo (n=14), rat recombinant GH (rrGH, n=8) or JI-38 (n=8; 50 μg / Kg / day), a potent GHRH-agonist. JI-38 did not elevate serum levels of GH or IGF-I, but markedly attenuated the degree of cardiac functional decline and remodeling after injury. In contrast, GH administration markedly elevated body weight, heart weight, circulating GH and IGF-I, but did not offset the decline in cardiac structure and function. Whereas, both JI-38 and GH augmented levels of cardiac precursor cell proliferation, only JI-38 increased anti-apoptotic gene expression. The receptor for GHRH was detectable on myocytes supporting direct activation of cardiac signal transduction. Collectively, these findings demonstrate that within the heart GHRH-agonists can activate cardiac repair following MI, suggesting the existence of a potential signaling pathway based on GHRH in the heart. The phenotypic profile of the response to a potent GHRH agonist has therapeutic implications.
Owner:MIAMI UNIVERISTY OF +1
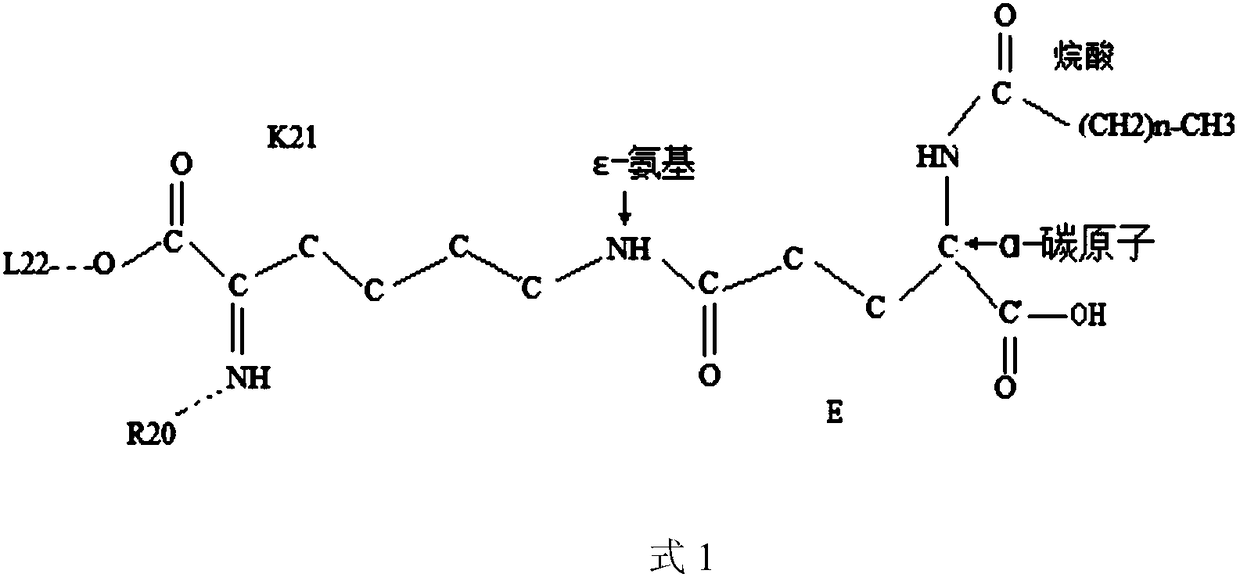
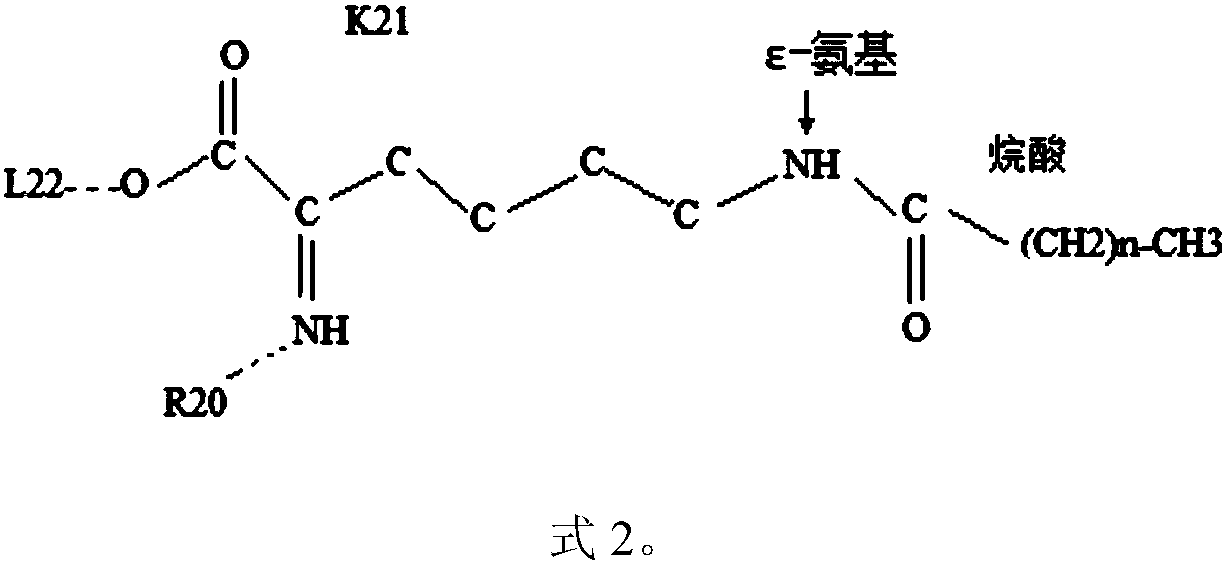
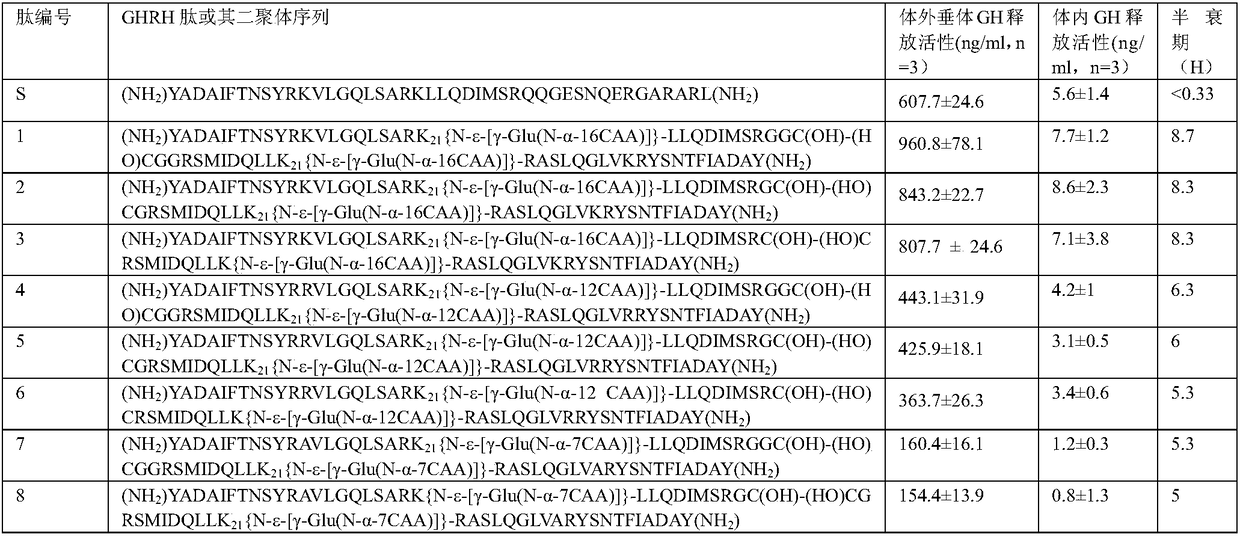
Novel growth hormone releasing hormone analogue peptide dimer and application thereof
ActiveCN109180800AEnhance release activityEasy to refactorNervous disorderPeptide/protein ingredientsHalf-lifeIn vivo
The invention discloses a novel growth hormone releasing hormone analogue peptide dimer and an application thereof. The amino acid sequence of the novel growth hormone releasing hormone analogue peptide dimer is as follows: (NH2)X1-ADAIFTNSYR-X12-VLGQLSAR-X21-LLQDIMS--C32-C32-theta-SMIDQLL-X21-RASLQGLV-X12-RYSNTFIADA-X1(NH2), wherein formula is R, RG or RGG; theta is GGR or GR and R or (NH2)X1-ADAIFTNSYR-X12-VLGQLSAR-X21-LLQDIMSRQQGESNQERGARAR--C46-C46-omega-RARAGREQNSEGQQRSMIDQLL-X21-RASLQGLV-X12-RYSNTFIADA-X1(NH2), wherein formula is L, LG or LGG and omega is GGL or GL and L. The growth hormone releasing hormone analogue peptide dimer can improve in vivo or in vitro growth hormone releasing activity and has a longer half life, so that the dimer has wide pharmacological activity, for example, promoting wound healing, promoting cardiac muscle cell reconstruction, improving sleeping quality, promoting proliferation and differentiation of generative cells so as to treat infertility, adjust immunity, reduce weight and treat diabetes.
Owner:深圳纳福生物医药有限公司
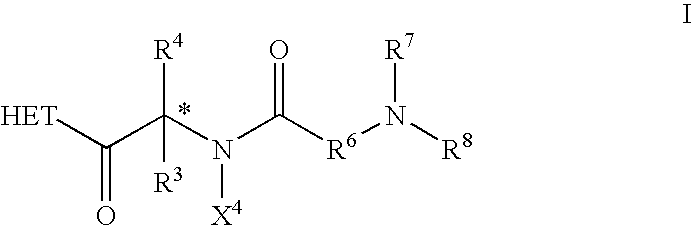
Dipeptide derivatives as growth hormone secretagogues
This invention is directed to compounds of the formula ##STR1## and the pharmaceutically-acceptable salts thereof, where the substituents are as defined in the Specification, which are growth hormone secretogogues and which increase the level of endogenous growth hormone. The compounds of this invention are useful for the treatment and prevention of osteoporosis and / or frailty, congestive heart failure, frailty associated with aging, obesity; accelerating bone fracture repair, attenuating protein catabolic response after a major operation, reducing cachexia and protein loss due to chronic illness, accelerating wound healing, or accelerating the recovery of burn patients or patients having undergone major surgery; improving muscle strength, mobility, maintenance of skin thickness, metabolic homeostasis or renal homeostasis. The compounds of the present invention are also useful in treating osteoporosis and / or frailty when used in combination with: a bisphosphonate compound such as alendronate; estrogen, premarin, and optionally progesterone; an estrogen agonist or antagonist; or calcitonin, and pharmaceutical compositions useful therefor. Further, the present invention is directed to pharmaceutical compositions useful for increasing the endogenous production or release of growth hormone in a human or other animal which comprises an effective amount of a compound of the present invention and a growth hormone secretagogue selected from GHRP-6, Hexarelin, GHRP-1, growth hormone releasing factor (GRF), IGF-1, IGF-2 or B-HT920. The invention is also directed to intermediates useful in the preparation of compounds of Formula I.
Owner:PFIZER INC
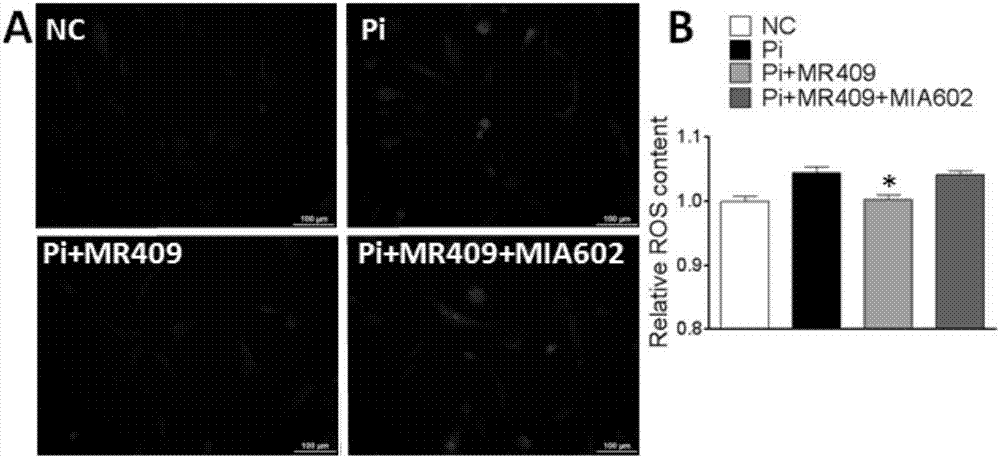
Applications of growth hormone release hormone agonist in preparation of anti-vascular calcification drugs
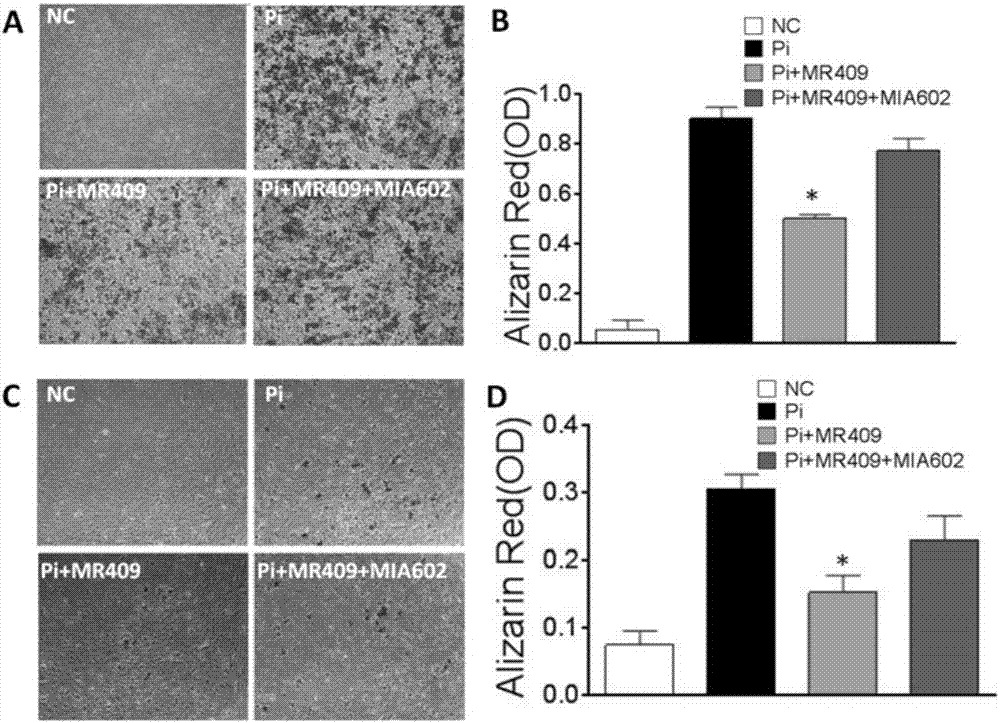
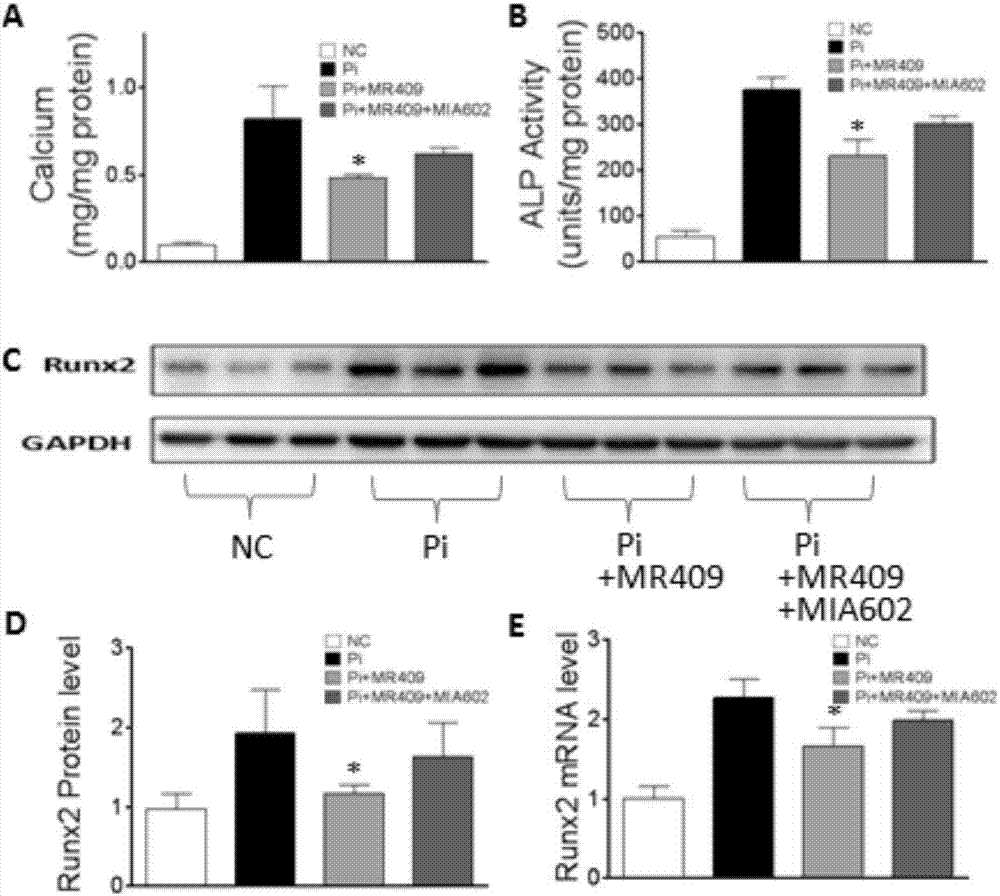
ActiveCN107320718APeptide/protein ingredientsCardiovascular disorderVascular calcificationGrowth hormone–releasing hormone
The present invention discloses a new medical use of a growth hormone release hormone (GHRH) agonist, particularly applications of a growth hormone release hormone agonist in preparation of anti-vascular calcification drugs. According to the present invention, GHRH-A can increase the cAMP level and the protein kinase A (PKA) activity in smooth muscle cells, can decrease the expression and the activity of NAPDH oxidase in smooth muscle cells, can decrease the ROS level in smooth muscle cells, can decrease the expression and the activity of Runx2 in smooth muscle cells, can decrease the expression and the activity of alkaline phosphatase (ALP) in cells, and can decrease the inorganic phosphorus content in blood so as to reduce the calcium deposition and the phosphorus deposition in aorta, reduce the activity of ALP in aorta, and achieve the purposes of treatment or prevention of vascular calcification and arteriosclerosis.
Owner:余红
Cardioprotective effects of ghrh agonists
InactiveUS20140057847A1Function increaseSimple structurePeptide/protein ingredientsDepsipeptidesAnti apoptotic genesFunctional decline
Disclosed herein are methods demonstrating that growth-hormone releasing hormone (GHRH) directly activates cellular reparative mechanisms within the injured heart, in a GH / IGF-I independent fashion. Following experimental myocardial infarction (MI), rats were randomly assigned to receive, during a 4 week period, either placebo (n=14), rat recombinant GH (rrGH, n=8) or JI-38 (n=8; 50 μg / Kg / day), a potent GHRH-agonist. JI-38 did not elevate serum levels of GH or IGF-I, but markedly attenuated the degree of cardiac functional decline and remodeling after injury. In contrast, GH administration markedly elevated body weight, heart weight, circulating GH and IGF-I, but did not offset the decline in cardiac structure and function. Whereas, both JI-38 and GH augmented levels of cardiac precursor cell proliferation, only JI-38 increased anti-apoptotic gene expression. Collectively, these findings demonstrate that within the heart, GHRH-agonists can activate cardiac repair following MI.
Owner:UNIV OF MIAMI +1
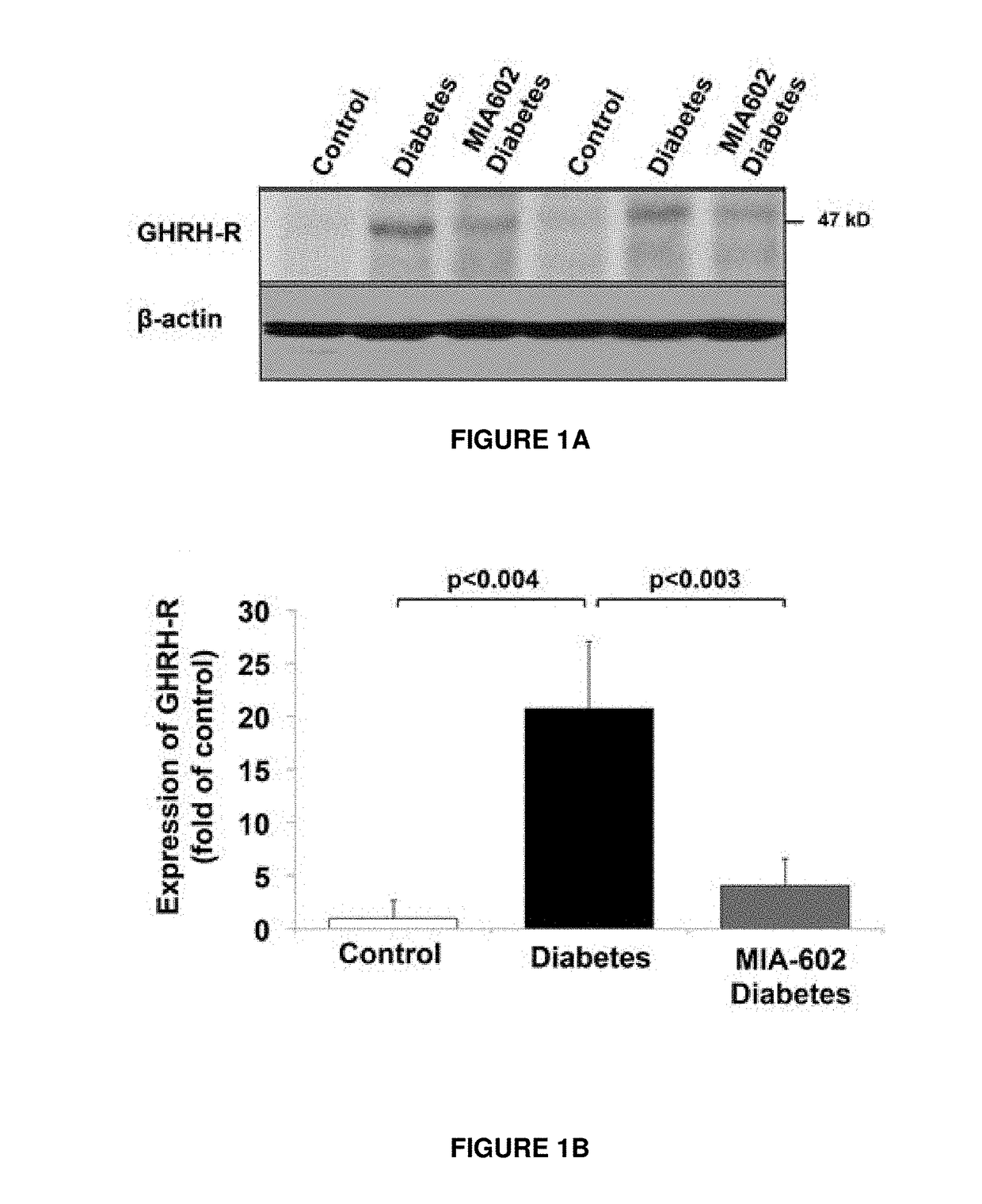
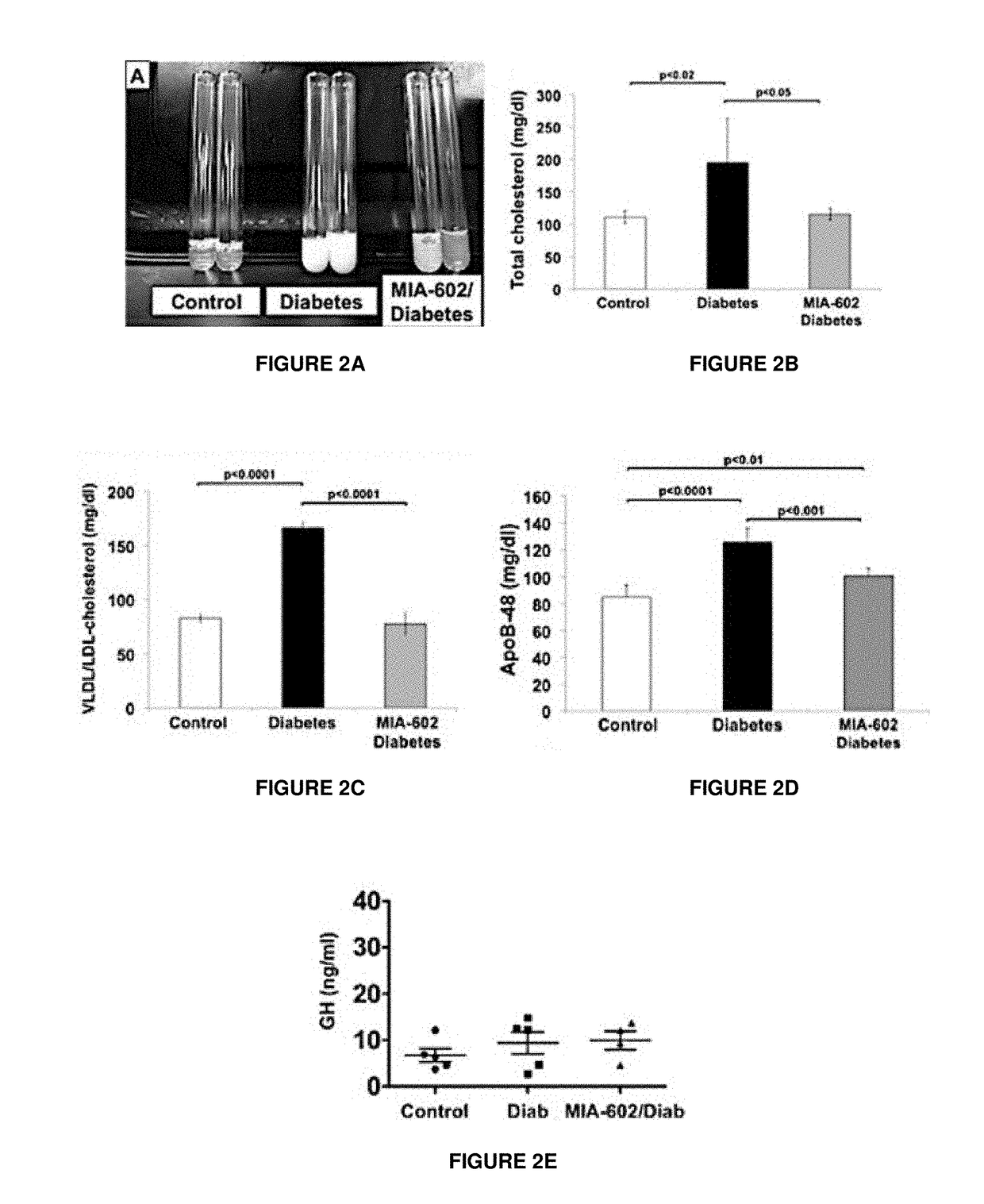
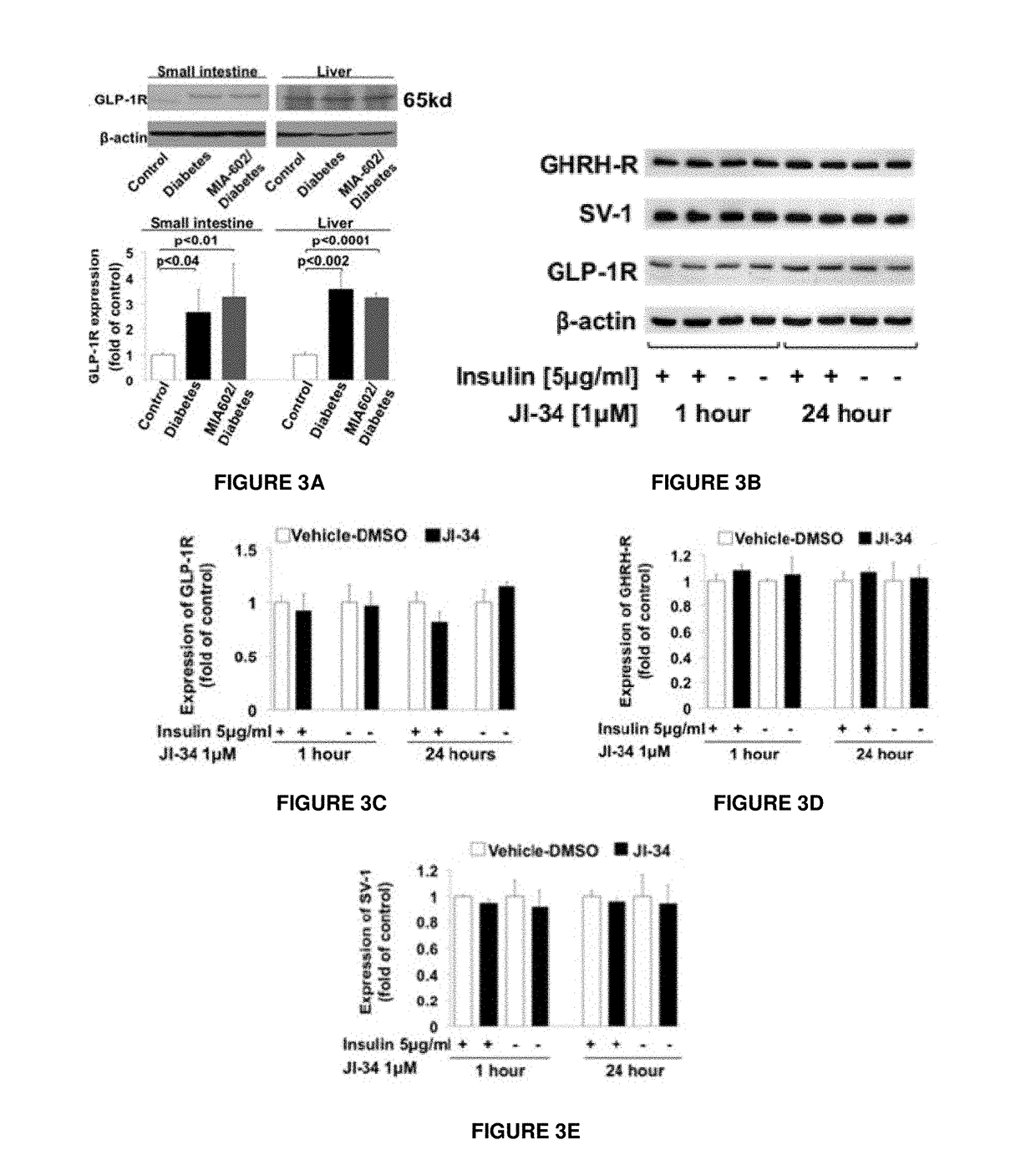
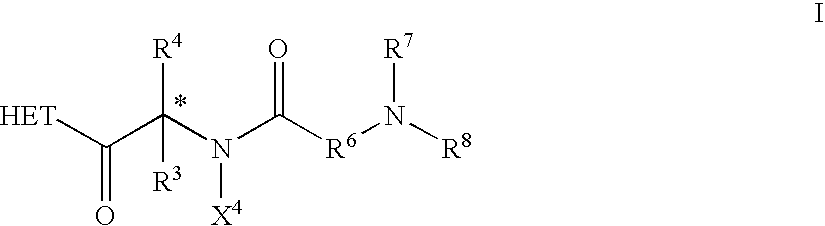
Materials and Methods of Treating Dyslipidemia
ActiveUS20170202907A1Effective treatmentPeptide/protein ingredientsMetabolism disorderDyslipidemiaBlood lipids
Described herein are materials and methods for treating dyslipidemia in a mammalian subject in need thereof comprising administering a growth hormone-releasing hormone (GHRH) antagonist or variant thereof to the subject.
Owner:UNIV OF MIAMI
Dipeptide derivatives
InactiveUS6924280B2Reduced responseReducing cachexia and protein lossBiocideNervous disorderProgesteronesConjugated estrogen
This invention is directed to compounds of the formula and the pharmaceutically-acceptable salts thereof, where the substituents are as defined in the Specification, which are growth hormone secretogogues and which increase the level of endogenous growth hormone. The compounds of this invention are useful for the treatment and prevention of osteoporosis and / or frailty, congestive heart failure, frailty associated with aging, obesity; accelerating bone fracture repair, attenuating protein catabolic response after a major operation, reducing cachexia and protein loss due to chronic illness, accelerating wound healing, or accelerating the recovery of burn patients or patients having undergone major surgery; improving muscle strength, mobility, maintenance of skin thickness, metabolic homeostasis or renal homeostasis. The compounds of the present invention are also useful in treating osteoporosis and / or frailty when used in combination with: a bisphosphonate compound such as alendronate; estrogen, conjugated estrogens, and optionally progesterone; an estrogen agonist or antagonist; or calcitonin, and pharmaceutical compositions useful therefor. Further, the present invention is directed to pharmaceutical compositions useful for increasing the endogenous production or release of growth hormone in a human or other animal which comprises an effective amount of a compound of the present invention and a growth hormone secretagogue selected from GHRP-6, Hexarelin, GHRP-1, growth hormone releasing factor (GRF), IGF-1, IGF-2 or B-HT920. The invention is also directed to intermediates useful in the preparation of compounds of Formula I.
Owner:PFIZER INC
Compounds with growth hormone releasing properties
Compounds of general formula (I), and their use for treating medical disorders resulting from a deficiency in growth hormone.
Owner:NOVO NORDISK AS
Modification and dimerization preparation and application of novel growth hormone releasing hormone similar peptide
ActiveCN111533800AEnhance release activityHigh activityNervous disorderPeptide/protein ingredientsDisulfide bondingDimer
The invention provides application of a molecular allosteric body and dimer of a human growth hormone releasing hormone (hGHRH) similar peptide in treating GH deficiency, infertility, senile dementiaand diabetes and enhancing immunity. The dimer is formed by connecting two identical hGHRH similar peptide monomers containing a single cysteine molecule allosteric structure through a disulfide bondformed by cysteine, and an H-shaped structure (intramolecular single Ser-to-Cys substitution) is formed. A side chain epsilon-amino of one lysine of the GHRH similar peptide is also subjected to fattyacid chain modification. Through fatty acid modification, the GH sustained release time of the GHRH similar peptide monomer or dimer is prolonged in vivo, and the longest release time reaches 17 days. The invention finds that the GHRH dimer with long-acting activity has an Aib-to-2Ala substitution structure, an 18C-to-18C disulfide bond formation structure, a C20 fatty acid chain modification structure and a C-terminal amidation structure.
Owner:深圳纳福生物医药有限公司
Compounds with growth hormone releasing properties
Owner:NOVO NORDISK AS
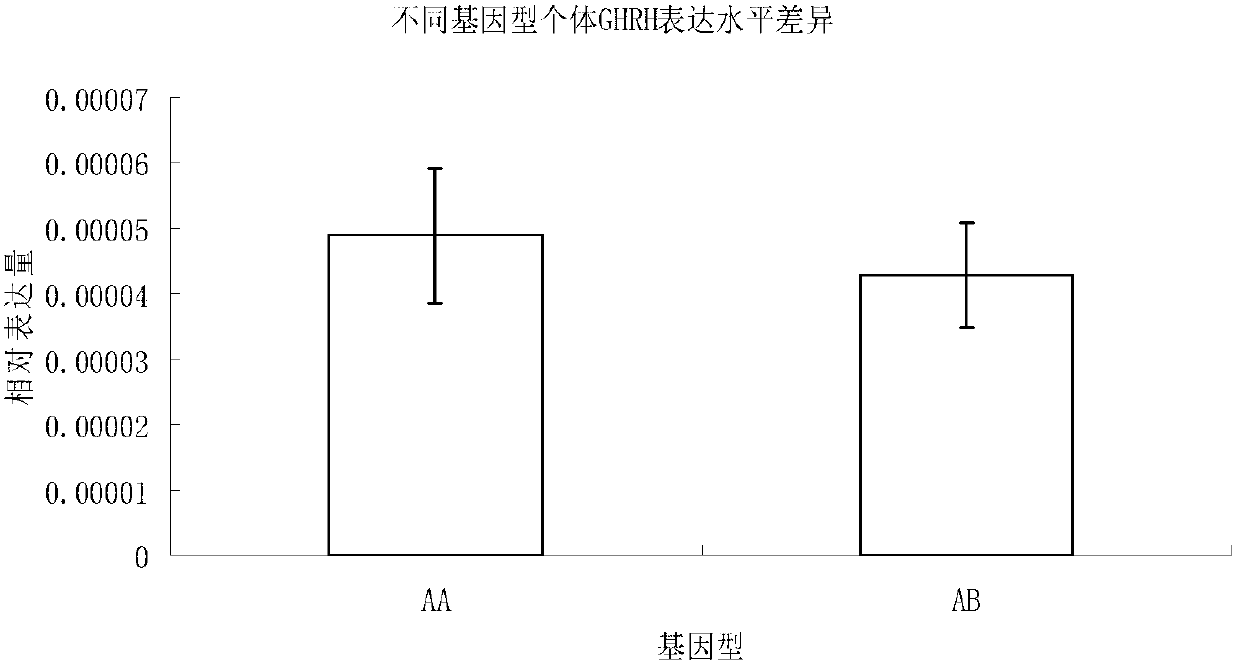
Molecular marker related to micropterus salmoides hatchability and application thereof
InactiveCN103103183AIncrease relative hatchabilityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationBiotechnologyGenotype
The invention discloses a molecular marker related to micropterus salmoides hatchability and an application thereof. The molecular marker related to micropterus salmoides hatchability is at the 97-163bp on GHRH (growth hormone releasing hormone) gene promoter sequence of the micropterus salmoides. A GHRH promoter gene with a 66bp sequence is named as A, and a deletion mutantion GHRH promoter gene is named as B. A primer is designed according to the GHRH promoter sequence of the micropterus salmoides, a parental genetype is identified through a molecular cone manner, a micropterus salmoides parent of AB genetype in production is removed, and a micropterus salmoides parent of AA genetype is maintained, so that the parental relative hatchability is improved. Compared with traditional molecular marker, the molecular marker has the advantages of strong purposiveness and direct action effect, is simple in operation, fast in detection, and low in detection cost, and can be widely promoted and used.
Owner:PEARL RIVER FISHERY RES INST CHINESE ACAD OF FISHERY SCI
Amantadine, memantine, and rimantadine conjugates and a pharmaceutical composition for treatment of neuronal disorders
A compound is provided that has the formula (II):where R1 in each occurrence is independently H, or C1-C4 alkyl; R2 is a nullity or CH—CH3, R3 is a nullity or C(O)—R6—NH; R6 is C2-C6 alkyl, (CH2CH2—O)n, or (CH(OH)CH2)n; n is an integer of between 1 and 4; R4 is a nullity or NH—R6—C(O); and R5 is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine, blood brain barrier (BBB) peptide, membrane translocating peptide, TAT peptides, endocannabinoids 1 & 2, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptide transferrin, glucosyl ester, lactic acid, leucine, tryptophan, glutamic acid.
Owner:MILLER LANDON C G
Analogs of human growth hormone-releasing hormone, their preparation and use
InactiveUS20060172927A1Prevention and therapyPeptide/protein ingredientsPeptide preparation methodsTert-Butyloxycarbonyl protecting groupSide chain
Growth hormone-releasing hormone analogs having the amino acid sequence of the formula: Dat-Ala-Asp-Ala-Ile-Phe-Thr-Asn-Ser-Tyr10-R11-R12-VAL-Leu-Ala-Gln-Leu-Ser-Ala-R20-R2-Leu-Leu-Gln-Asp-Ile-Nle-Asp-R29-NH2 (I), wherein R11 is hArg, Gab or Gap; R12 is hArg, Orn, Gab or Gap; R20 is hArg, hArg, Gab or Gap; R21 is hArg, Orn, Gab or Gap; R29 is D-Arg, hArg, Gab or Gap; and their pharmaceutically acceptable salts. Said peptides are potent and selective stimulators of GH release and they are highly resistant to enzymatic degradation. Said peptides are prepared using the solid phase synthesis method, by introducing suitable derivatives of lysine, 2,4-diaminobutyric acid or 2,3-diaminopropionic acid at appropriate positions in the peptide chain attached to a polymeric support, deprotecting side-chain amino gorups and reacting free amino groups with a guanidinating agent, removing all remaining t-butyloxycarbonyl protective groups, and cleaving the synthesized peptide from the support, followed by purification and optionally, converting the peptide into a pharmaceutically acceptable salt.
Owner:INSTITUT FARMACEUTYCZNY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com