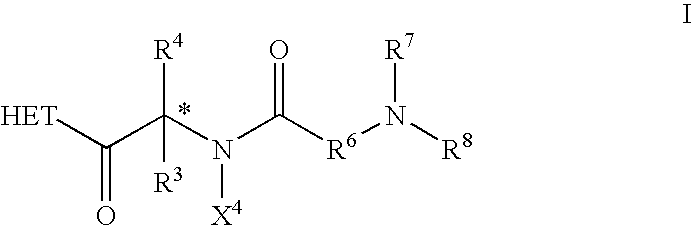
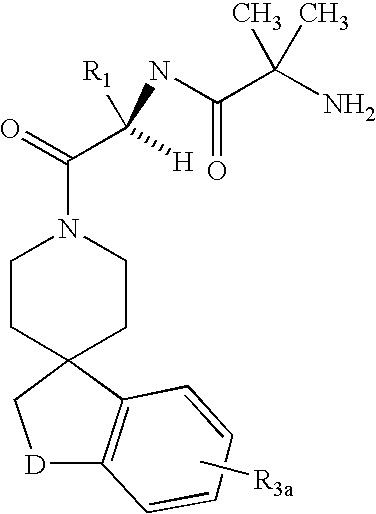
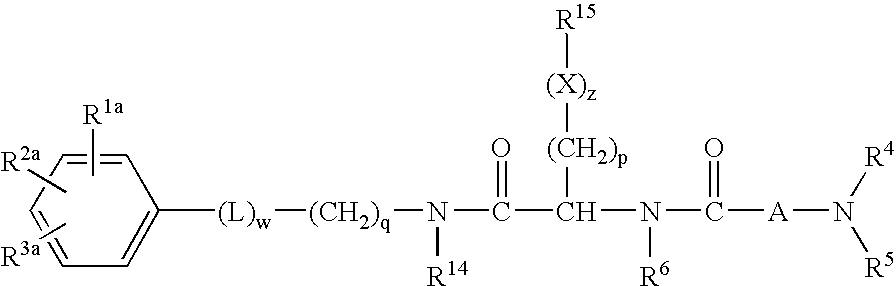
Dipeptide derivatives as growth hormone secretagogues
a technology of growth hormone and dipeptide, which is applied in the direction of peptides, drug compositions, metabolic disorders, etc., can solve the problems of high price, disease associated with the source of pituitary gland could be transmitted to the recipient of growth hormone, and still a very expensive product, so as to promote the release of endogenous growth hormone, improve muscle strength, and maintain skin thickness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
2-Amino-N-[2-(8a(S)-benzyl-3-oxo-tetrahydro-oxazolo[3,4-a]pyrazin-7-yl)-1(R )-(3,5-dichloro-benzyloxymethyl)-2-oxo-ethyl]-2-methyl-propionamide, hydrochloride
##STR85##
A. 2-Benzyl-piperazine-1,2,4-tricarboxylic acid 1-benzyl ester 4-tert-butyl ester 2-methyl ester
To a stirred solution of piperazine-1,2,4-tricarboxylic acid 1-benzyl ester 4-tert-butyl ester 2-methyl ester (20.0 g, 53 mmol), prepared as described by Bigge et al. (Tetrahedron Let. 1989, 30, 5193), in tetrahydrofuran (500 mL) was added N,N-dimethylformamide (50 mL). The reaction was cooled to about -78.degree. C., and a 1M solution of sodium bis (trimethylsilyl)amide in tetrahydrofuran (80 mL) was added. The reaction was stirred at about -78.degree. C. for about 1 hour, and then benzyl bromide (9.4 mL, 79 mmol) was added. The reaction was stirred for about 30 minutes more at about -78.degree. C., then warmed to room temperature and stirred overnight. The reaction was quenched with saturated sodium bicarbonate solution, a...
example 2
2-Amino-N-[2-(8a(S)-benzyl-2-methyl-3-oxo-hexahydro-imidazol[1,5-a]pyrazin- 7-yl)-1(R)-benzyloxymethyl-2-oxo-ethyl]-2-methyl-propionamide, hydrochloride
##STR86##
A. 3-Benzyl-piperzaine-1,3-dicarboxylic acid 1-tert-butyl ester 3-methyl ester
The title compound of part 1-A (2.80 g, 5.98 mmol) was deprotected according to the method described in General Procedure F to give the title compound of part 2-A as a white foam (1.89 g, 95%): +APcl MS (M+1).sup.+ 335, (M-55).sup.+ 279, (M-99).sup.+ 235; .sup.1 H NMR=400 MHz (CDCl.sub.3) .delta.: 7.28-7.18 (arom, m, 5H), 3.66 (Me, s, 3H), 1.40 (BOC, s, 9H).
B. 3,4-Dibenzyl-piperazine-1,3-dicarboxylic acid 1-tert-butyl ester 3-methyl ester
To a solution of the title compound of part 2-A (1.22 g, 3.65 mmol) and diisopropylamine (0.63 mL, 3.65 mmol) in acetonitrile (18 mL) was added benzyl bromide (0.45 mL, 3.83 mmol), and the reaction was stirred at room temperature overnight. The reaction was then heated to reflux for about 5 hours. The solvent was t...
example 3
2-Amino-N-{1(R)-benzyloxymethyl-2-[1,3-dioxo-8a(S)-pyridin-2-ylmethyl-2-(2, 2,2-trifluoro-ethyl)-hexahydro-imidazo[1,5-a]pyrazin-7yl]-2-oxo-ethyl}-2-me thyl-propionamide, hydrochloride
##STR87##
A. 2-Pyridin-2-ylmethyl-piperazine-1,2,4-tricarboxylic acid 1-benzyl ester 4-tert-butyl ester 2-methyl ester:
A stirred solution of piperazine-1,2,4-tricarboxylic acid 1-benzyl ester 4-tert-butyl ester 2-methyl ester (200 g, 529 mol), prepared as described by Bigge et al. (Tetrahedron Let. 1989, 30, 5193), in tetrahydrofuran (200 mL) and DMF (1.5 L) was cooled to about -78 C., and a 0.5M solution of potassium bis(trimethylsilyl)amide in THF (1.27 L) was added. After the above solution had stirred for about one hour, the free base of 2-picolyl chloride was generated by extracting the corresponding hydrochloride salt (217 g, 1.32 mol) from saturated sodium bicarbonate solution with methylene chloride. The combined organic extracts were dried (MgSO.sub.4), concentrated, immediately dissolved in DM...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com