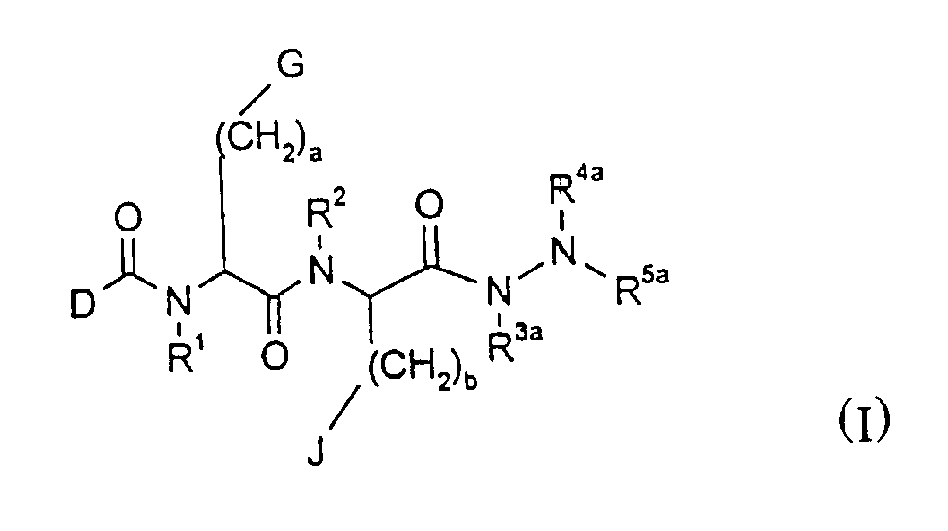
Compounds with growth hormone releasing properties
A compound, halogen technology, applied in the application field of medical diseases, can solve problems such as invalidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0176] (2E)-5-Amino-5-methylhex-2-enoic acid N-((1R)-1-(N-[(1R)-2-(N'-acetylhydrazino)-1-benzyl Base-2-oxoethyl]-N-methylcarbamoyl)-2-(2-naphthyl)ethyl)-N-methylamide
[0177] tert-Butyl N'-Acetylhydrazinocarboxylate
[0178] To a solution of tert-butyl carbazate (1.0 g, 7.6 mmol) and pyridine (3.1 ml) in dichloromethane (5 ml) was added acetic anhydride (1.5 ml) slowly and the mixture was stirred overnight. The mixture was added to dichloromethane (50ml) and washed with water (2 x 10ml) and brine (10ml) and dried (MgSO 4 ), filtered and concentrated in vacuo to afford 0.95 g of tert-butyl N'-acetylhydrazinecarboxylate as a yellow oil.
[0179] LC-MS:R t =5.39 minutes, m / z=349.6(m+1)
[0180] 1 H NMR (CDCl 3 ) Selected peak: δ1.5(s, 9H, (CH 3 ) 3 C-O); 2.05(s,3H,CH 3 CO)
[0181] N-[(1R)-2-(N'-acetylhydrazino)-1-benzyl-2-oxoethyl]-N-methylcarbamate tert-butyl ester
[0182] tert-Butyl N'-acetylhydrazinecarboxylate (0.95g, 5.45mmol) was dissolved in dichloromet...
Embodiment 2
[0200] (2E)-5-amino-5-methylhex-2-enoic acid N-((1R)-1-(N-[(1R)-2-(N'-acetyl-N-methylhydrazino )-1-benzyl-2-oxoethyl]-N-methylcarbamoyl)-2-(2-naphthyl)ethyl)-N-methylamide
[0201] N'-Acetyl-N-methylhydrazine carboxylate tert-butyl ester
[0202] To a solution of tert-butyl N-methylhydrazinecarboxylate (0.62g, 4.20mmol) dissolved in dichloromethane (10ml) was added acetic anhydride (0.79ml, 8.40mmol) and pyridine (1.36ml, 16.80mmol) And the mixture was stirred overnight. Dichloromethane (50ml) was then added and the mixture was washed with water (3 x 10ml), dried (MgSO 4 ), filtered and concentrated in vacuo to afford 0.32 g (41%) of tert-butyl N'-acetyl-N-methylhydrazinecarboxylate as an oil.
[0203] 1 H NMR (CDCl 3 ) Selected peak: δ1.45+1.48(2s,9H,(CH 3 ) 3 C-O, rotamers); 1.98(s,3H,COCH 3 ); 3.14+3.17(2s,3H,N-CH 3 , rotamer) N'-methylhydrazine acetate
[0204] To a solution of tert-butyl N'-acetyl-N-methylhydrazinecarboxylate (0.3 g, 1.59 mmol) in dichloro...
Embodiment 3
[0224] (2E)-5-Amino-5-methylhex-2-enoic acid N-((1R)-1-(N-[(1R)-2-(N'-acetyl-N'-methylhydrazine Base)-1-benzyl-2-oxoethyl]-N-methylcarbamoyl)-2-(2-naphthyl)ethyl)-N-methylamide
[0225] tert-Butyl N'-benzylidenehydrazinecarboxylate
[0226] To a solution of tert-butyl carbazate (10.0 g, 75.64 mmol) in 99% ethanol (100 mL) was added benzaldehyde (7.64 mL, 75.64 mmol) and the mixture was stirred for 60 minutes. The mixture was cooled to 0°C, filtered and the precipitate was washed with cold ethanol and dried to give 13.47 g (81%) of tert-butyl N'-benzylidenehydrazinecarboxylate as white crystals.
[0227] Mp 184-186°C
[0228] 1 H NMR (CDCl 3 ):δ1.52(s,9H,(CH 3 )C); 7.34-7.92 (m, 7H, aromatic hydrogen)
[0229] tert-butyl N'-benzylidene-N-methylhydrazinecarboxylate
[0230] To a solution of tert-butyl N'-benzylidenehydrazinecarboxylate (2.0g, 9.07mmol) dissolved in anhydrous tetrahydrofuran (20ml) was added iodomethane (4.54ml, 72.6mmol) and the solution was cooled ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com