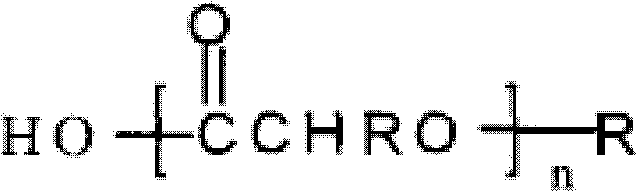Preparation method of medicated slow-release degradable bone scaffold
A bone scaffold and slow-release technology, applied in the field of medicine, can solve problems such as adverse reactions, liver and kidney damage, etc., and achieve the effects of strong antibacterial effect, enhanced curative effect, and delayed drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
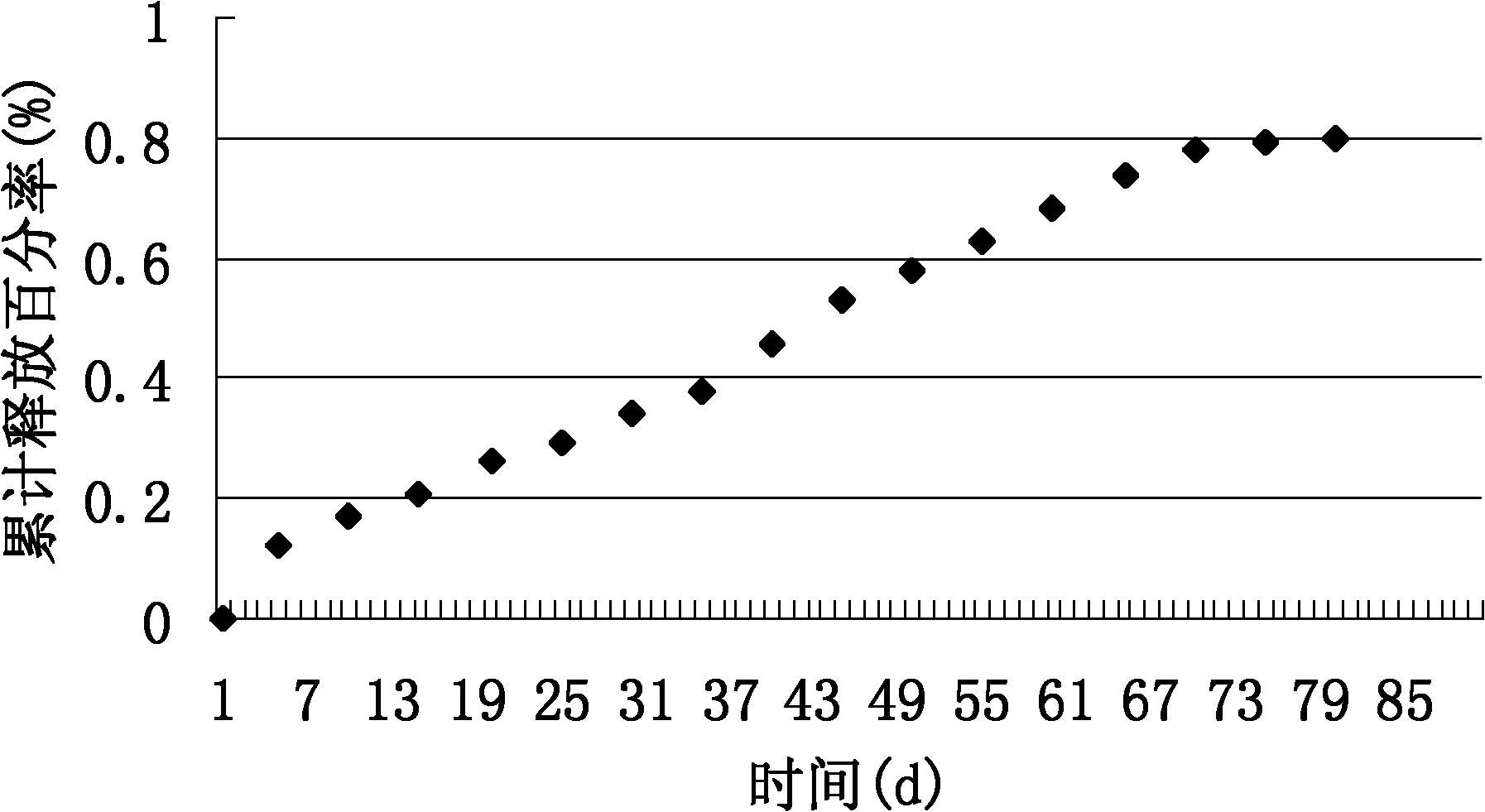
Embodiment 1
[0034] (1) 2g PLGA (molar ratio of lactide to glycolide is 50:50) is dissolved in 100 mL of dichloromethane to form an oil phase.
[0035] (2) 1g rifampicin is dissolved in 20ml double distilled water to form a 0.05g / ml drug solution.
[0036] (3) Prepare 1200 ml of a polyvinyl alcohol aqueous solution with a concentration of 1.5% by mass as the outer water phase.
[0037] (4) Pour the drug solution of step (2) into the oil phase of step (1), mix at 0°C and sonicate for 3 minutes under ice bath, and emulsify uniformly to obtain a W / O primary emulsion.
[0038] (5) Take 1200 mL of an aqueous polyvinyl alcohol solution with a concentration of 1.5% by mass. Pour the W / O primary emulsion obtained in step (4) evenly into it. Then add 3 drops of emulsifier Tween80. Stir at high speed and emulsify evenly to obtain W / O / W double emulsion.
[0039] (6) Stir the W / O / W double emulsion at room temperature for 24 hours to volatilize the organic solvent until the microspheres are precipitated and har...
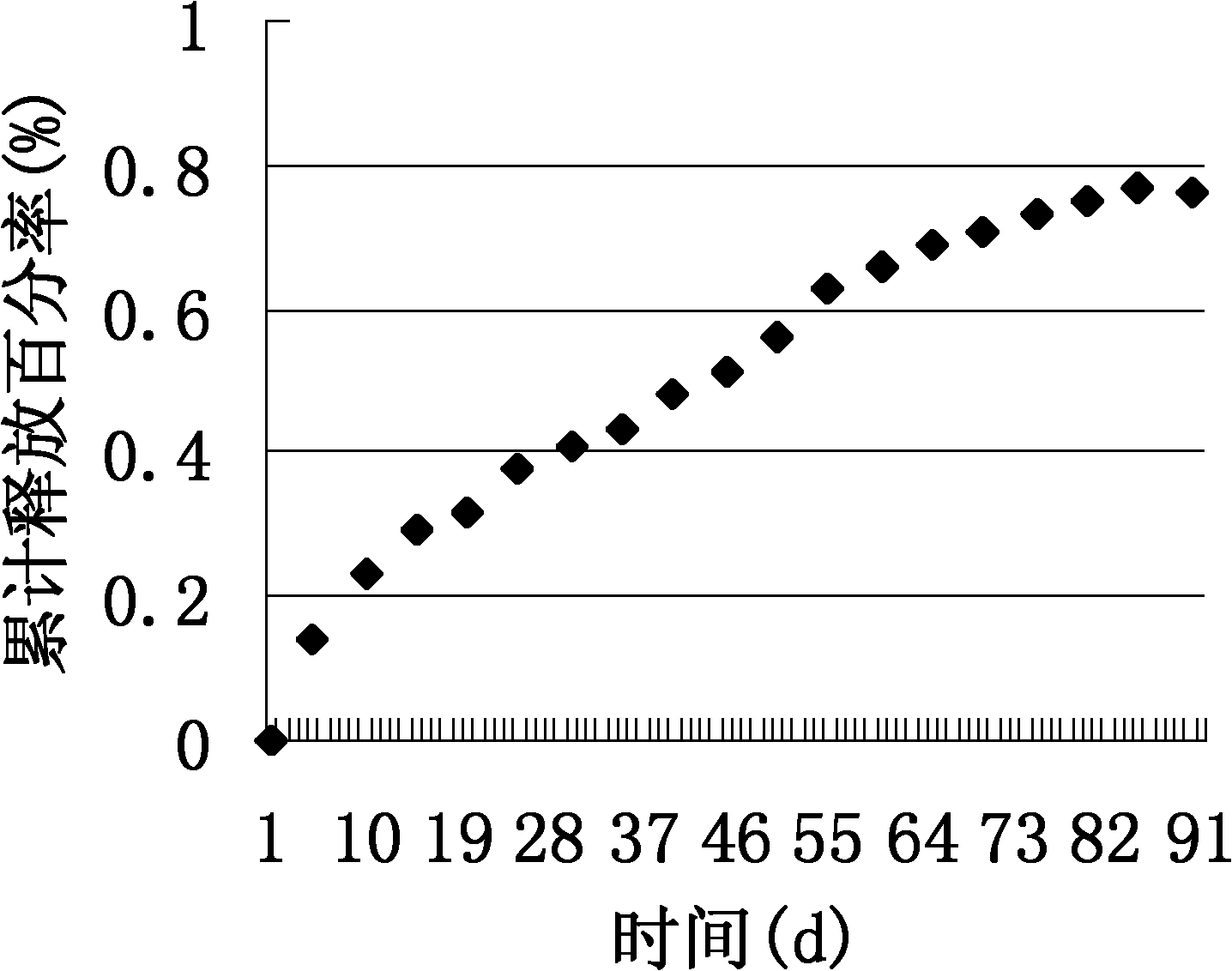
Embodiment 2
[0044] (1) 1 g PLGA (mole ratio of lactide to glycolide is 75:25) is dissolved in 70 mL of dichloromethane to form an oil phase.
[0045] (2) 0.7g of isoniazid is dissolved in 10ml of double-distilled water to form a drug solution of 0.07g / ml.
[0046] (3) Prepare 600 ml of a polyvinyl alcohol aqueous solution with a concentration of 2% by mass as the outer water phase.
[0047] (4) Pour the drug solution of step (2) into the oil phase of step (1), mix at 0°C and sonicate for 3 minutes under ice bath, and emulsify uniformly to obtain a W / O primary emulsion.
[0048] (5) Take 600 mL of an aqueous solution of polyvinyl alcohol with a concentration of 2% by mass. Pour the W / O primary emulsion obtained in step (4) evenly into it. Then add 3 drops of emulsifier Tween80. Stir at high speed and emulsify uniformly to obtain W / O / W double emulsion.
[0049] (6) Stir the W / O / W type double emulsion at room temperature for 24 hours to volatilize the organic solvent until the microspheres are precip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com