Method for coaxially preparing injectable PLGA drug-carrier microsphere by utilizing electrospinning machine
A technology of electrospinning machine and drug-loaded microspheres, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of large toxic and side effects, and achieve biocompatibility Good, great research value and development prospect, realize the effect of slow and long-term release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] ① Weigh 60mg PLGA and dissolve it in 1ml hexafluoroisopropanol to prepare a 6% (g / ml) shell layer PLGA solution, stir magnetically for 4 hours at room temperature; prepare a certain concentration of PTX and ETP solutions with dichloromethane solution as Nuclei layer solution, vortexed to mix.
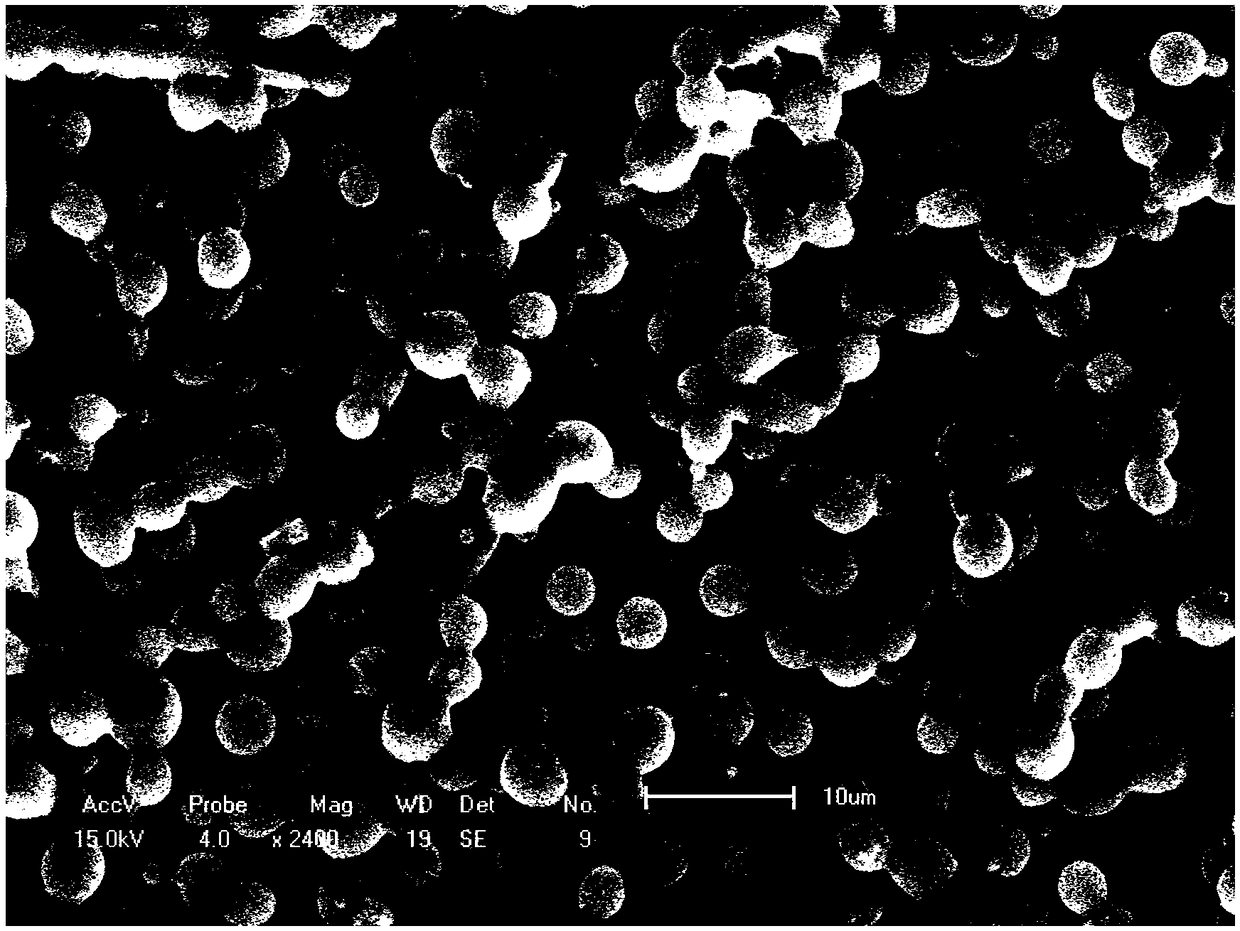
[0029] 2. Adjust the spinning voltage of electrospinning to be 10KV, the spinning distance to be 20cm, and the spinning speed shell layer and core layer to be 1.075ml / h and 0.12ml / h respectively, and then freeze-dry to obtain PLGA drug-loaded microspheres. Store at -20°C for later use. The scanning electron microscope results are as follows figure 1 As shown, the average particle size ranges from 1 to 4 μm, and the shape of the microspheres is relatively regular and there is adhesion.
Embodiment 2
[0031] ①Weigh 50mg of PLGA and dissolve it in 1ml of hexafluoroisopropanol to prepare a 5% (g / ml) PLGA solution, stir magnetically at room temperature for 4 hours to obtain a uniform and stable shell solution; use dichloromethane solution to prepare a certain concentration The PTX and ETP solutions were vortexed to obtain the core layer solution.
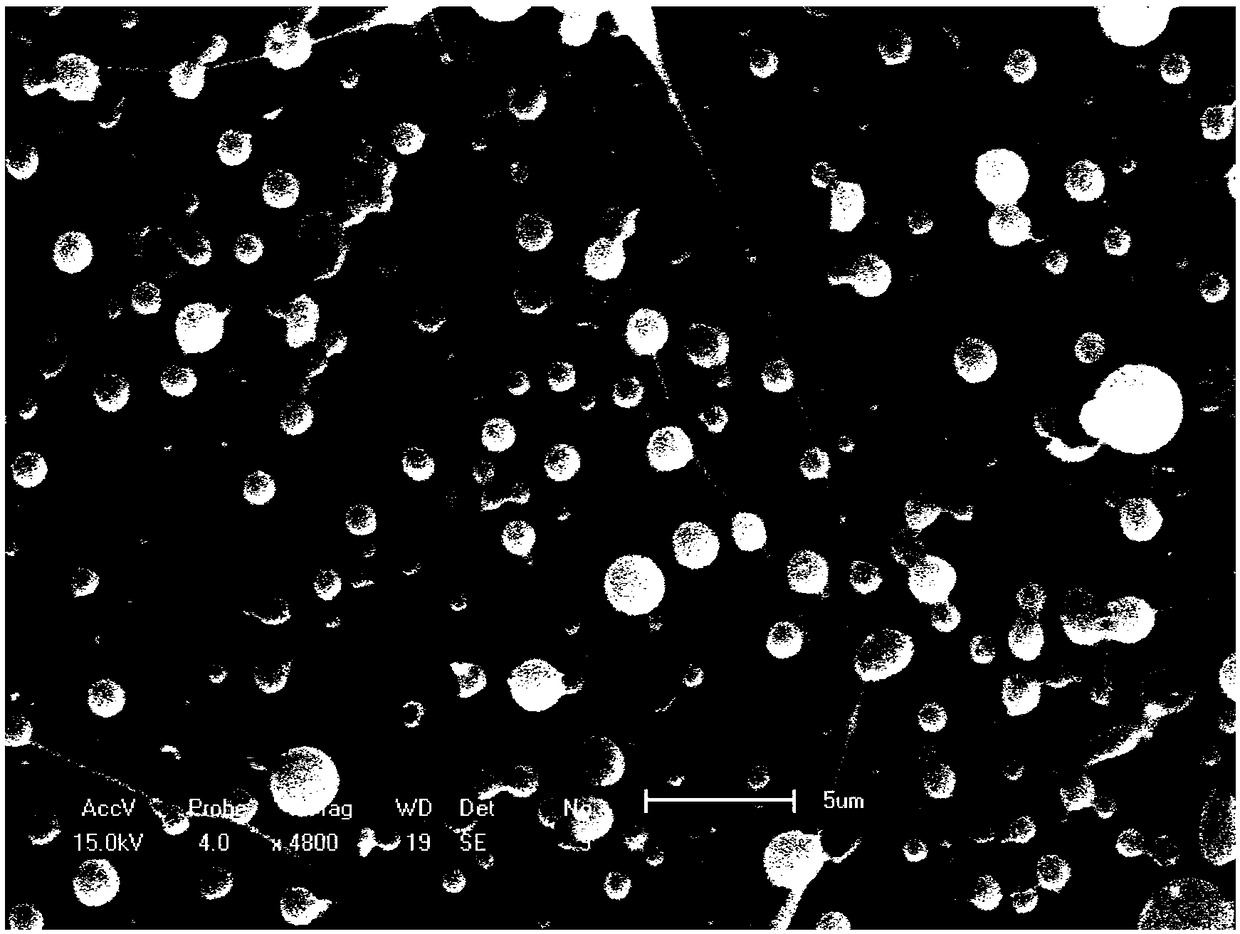
[0032] ② Adjust the spinning voltage of the electrospinning machine to 15KV, the spinning distance to 15cm, and the spinning speed to 1.075ml / h and 0.12ml / h for the shell layer and the core layer, respectively, and obtain PLGA drug-loaded microspheres by high-voltage electrospinning, and freeze Store at -20°C after drying. The electron microscope results are as figure 2 As shown, the particle size ranges from 1 to 3.5 μm, the size is relatively uneven, and there are filaments.
Embodiment 3
[0034] ①Weigh 50mg of PLGA and dissolve in 1ml of hexafluoroisopropanol solution to prepare a 5% (g / ml) PLGA solution, and stir magnetically for 4 hours at room temperature to obtain a uniform and stable shell solution; Concentration of PTX and ETP solution, after vortex mixing to obtain the core layer solution.
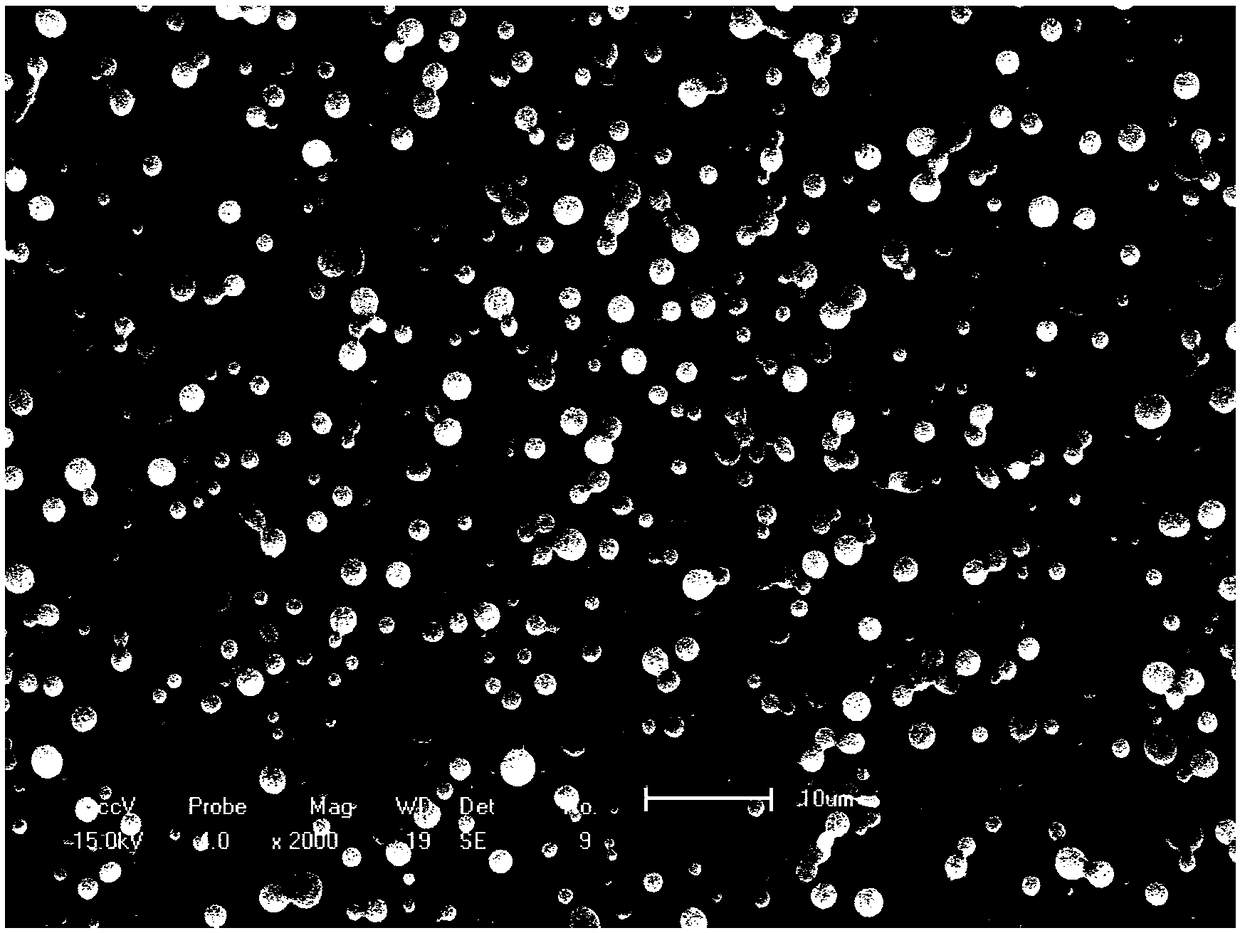
[0035] ② Adjust the spinning voltage of the electrospinning machine to 18KV, the spinning distance to 15cm, and the spinning speed to 1.075ml / h and 0.12ml / h for the shell layer and the core layer, respectively, and obtain PLGA drug-loaded microspheres by high-voltage electrospinning, and freeze Store at -20°C after drying. The electron microscope results are as image 3 As shown, the shape is relatively regular.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com