Nerve catheter as well as preparation method and application thereof
A technology for nerve conduits and neurotrophic factors, applied in conduits, textiles and papermaking, conjugated synthetic polymer man-made filaments, etc., can solve the problems of reduced activity and loss of biologically active substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The preparation method of nerve guide provided by the present invention comprises the following steps:
[0044] (1) Preparation of core layer solution: one or more substances with stabilizing effect and bioactive components are simultaneously dissolved in phosphate buffer under sterile conditions to obtain core layer solution; The substance is selected from: bovine serum albumin (BSA), fibrin; preferably, the following filling substances can be added in this step, so that the viscosity of the obtained core layer solution is moderate, and the moderateness refers to meeting the requirements of electrospinning ;
[0045] (2) Preparation of the shell solution: dissolve the biodegradable material in a suitable strong volatile organic solvent, stir until completely dissolved, and obtain a shell solution with moderate viscosity; the strong volatile organic solvent is selected from the following One or more of: trifluoroethanol, acetone, N,N-dimethylformamide, hexafluoroisopro...
Embodiment 1
[0062] Preparation of Nerve Conduit I
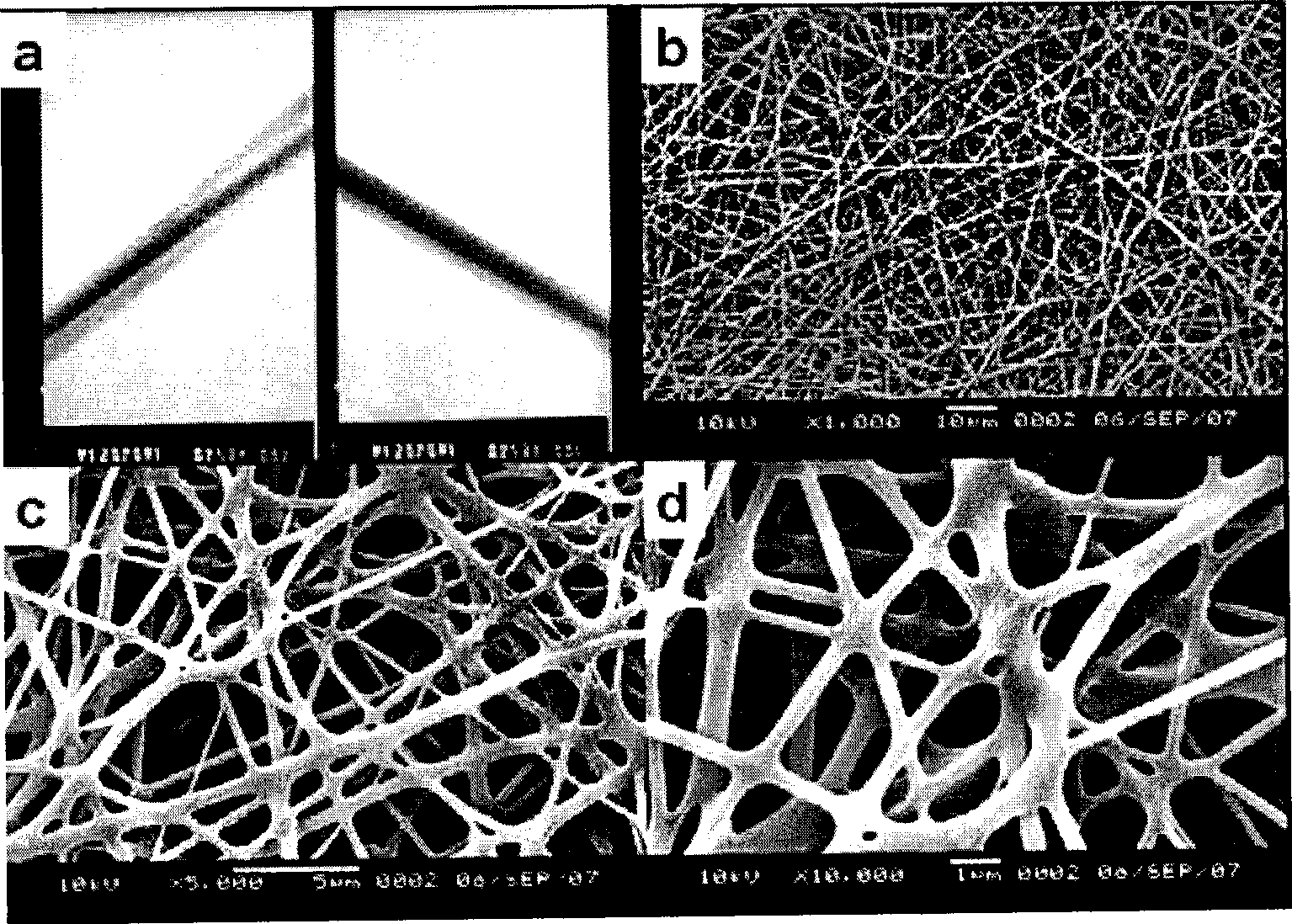
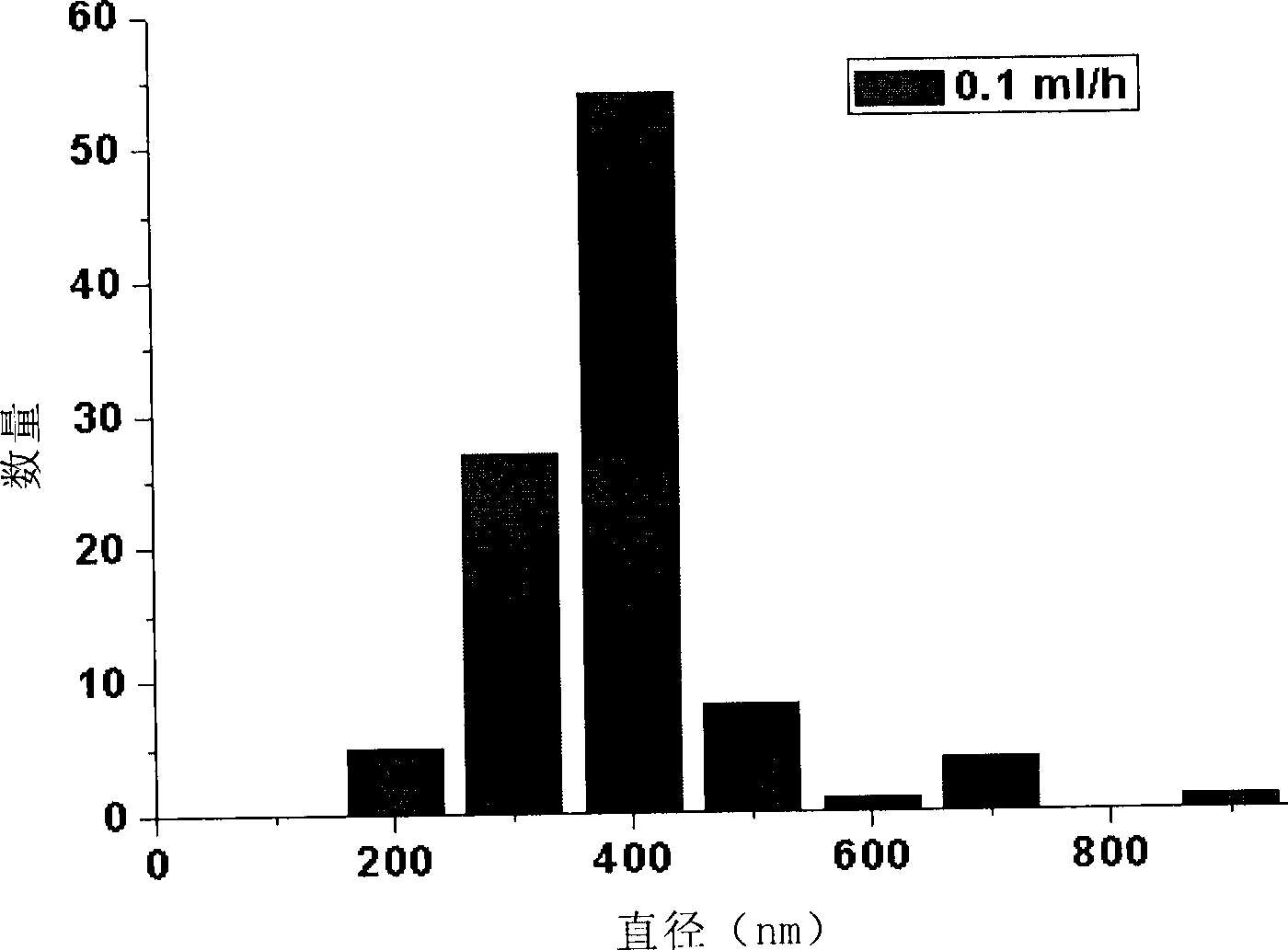
[0063] Weigh 10ug of human recombinant NGF freeze-dried powder with an electronic balance and dissolve it in 5mL of PBS to obtain a 2ug / mL NGF solution containing 100mg / mL of BSA. Take 2.5mL of 2ugmL NGF solution, dilute it into 2.5mL of PBS (pH value is 7.2) solution, add 485mg of BSA (as filling protein in the spinning process), stir and mix with a magnetic bar for 2 hours, and get 5mL with a concentration of 1ug NGF solution per mL was used as the inner layer solution; 600 mg of P(LLA-CL) (a copolymer of lactic acid and caprolactone 1:1) was weighed with an electronic balance, dissolved in 10 mL of trifluoroethanol, and stirred with a magnetic bar for 2 Hours, 10 mL of P(LLA / CL) solution was obtained as the outer layer solution. The coaxial electrospinning device consists of a high-voltage electrostatic generator, 2 injection pumps, inner and outer spray needles and receiving guide needles. The inner diameter of the inner nozzle of ...
Embodiment 2
[0065] Prepare Nerve Conduit II
[0066] Weigh 10ug human recombinant CNTF and 600mg P(LLA-CL) with an electronic balance, according to the preparation steps of Example 1, prepare a 5mL CNTF solution with a concentration of 1ug / mL as the inner layer solution, and 10mL LP(LA / CL) solution as the outer solution. Then spin according to the spinning steps and parameters of Example 1 to obtain a P(LLA- CL) / BSA / CNTF composite nerve conduit II, the wall thickness of the nerve conduit II is 0.3-0.4mm, the porosity is above 85%, its mechanical strength is that the average tensile strength is greater than 4MPa, and the plastic deformation index under physiological conditions is less than 15 %.
PUM
| Property | Measurement | Unit |
|---|---|---|
| breaking strength | aaaaa | aaaaa |
| breaking strength | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com