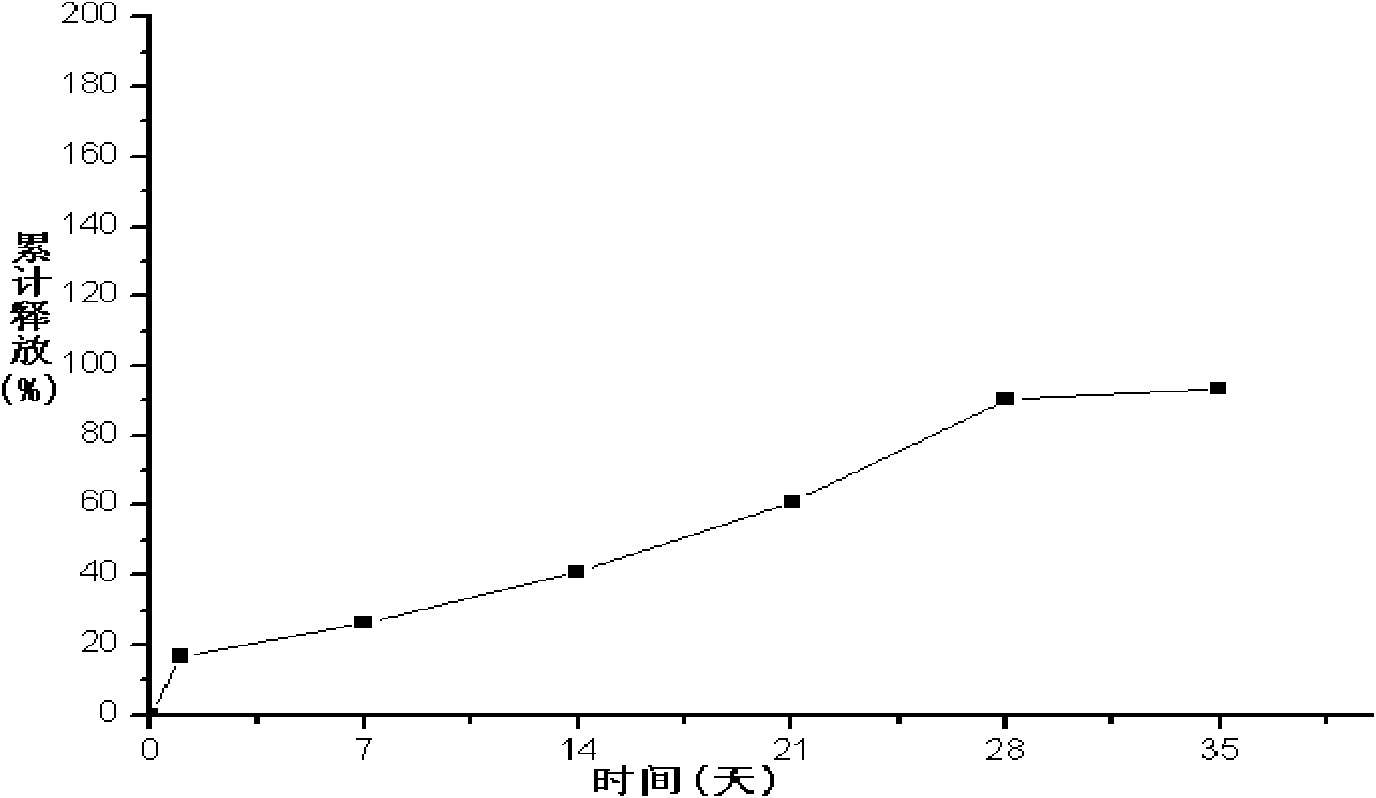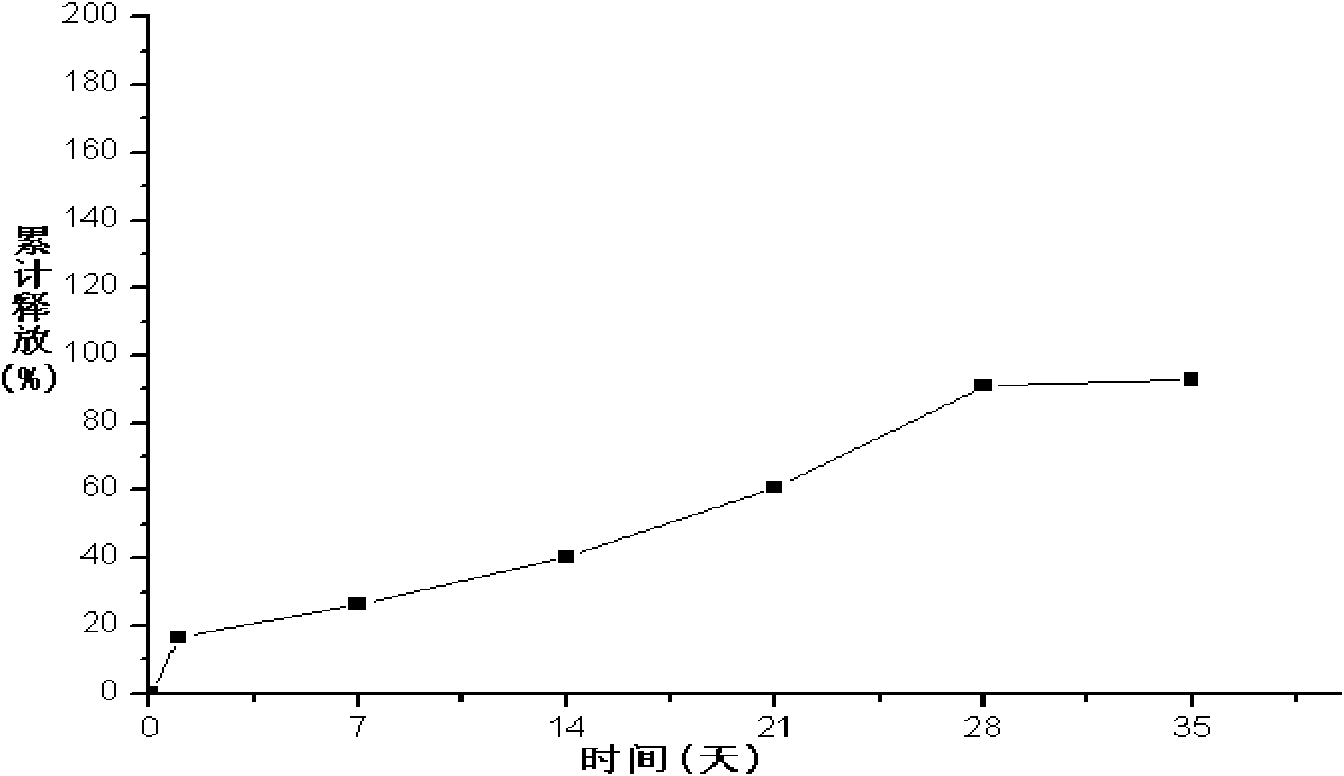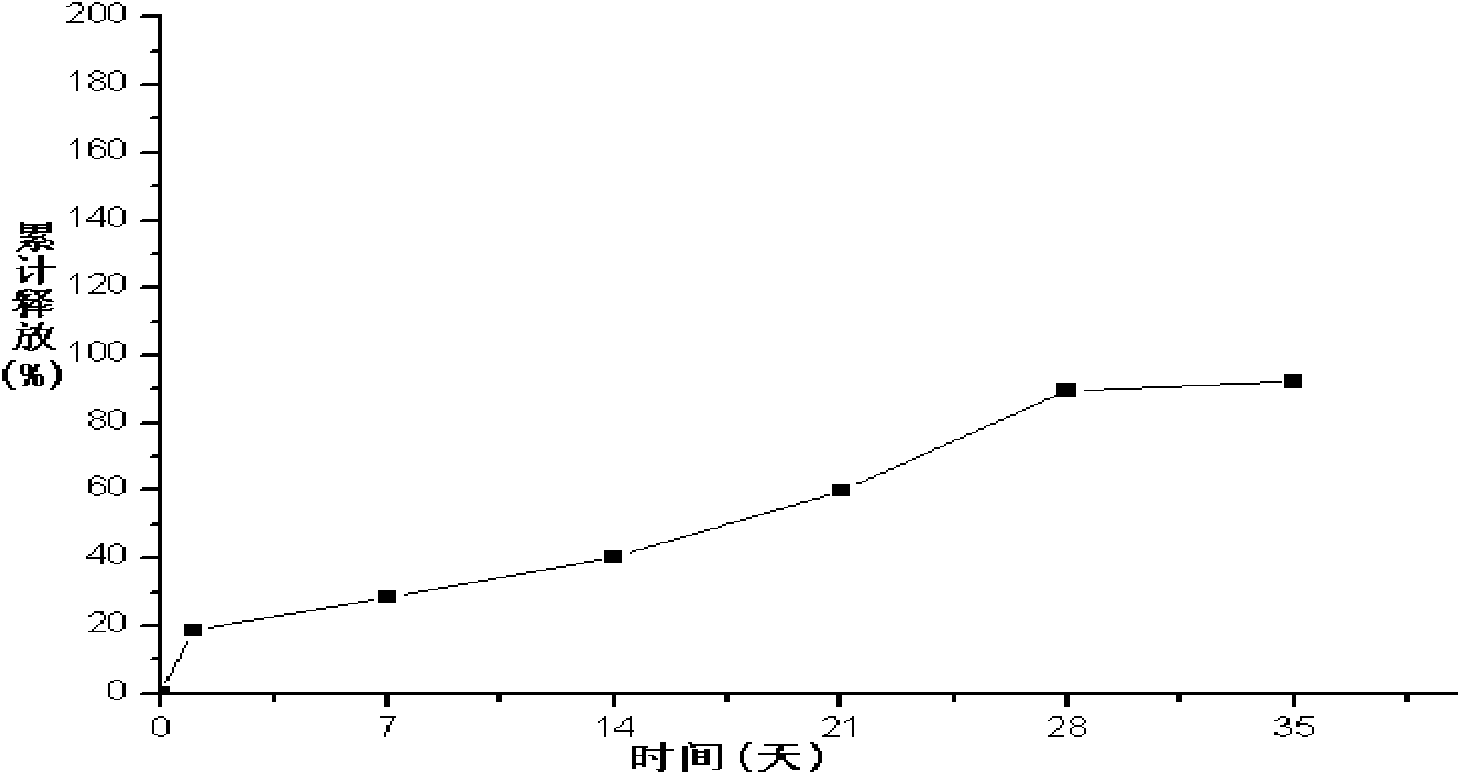Liraglutide long-acting microsphere injection and preparation method thereof
A technology of liraglutide and injection, which is applied in the field of liraglutide long-acting microsphere injection and its preparation, can solve the problems of long half-life, no industrial production, and inability to guarantee rapid release of drugs, etc., to reduce treatment costs , Improve medication compliance and reduce treatment burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] 1. Weigh 400mg of liraglutide, add it to 80ml of water for injection, and heat it on a water bath at 45°C to completely dissolve the drug as the inner water phase;
[0075] 2. Weigh 4 g of PLGA (75 / 25, molecular weight: 10,000 Daltons), add 100 ml of acetone to completely dissolve PLGA (75 / 25), and use it as the organic phase;
[0076] 3. Add the organic phase of step 2 to the inner water phase of step 1, mix the inner water phase and the organic phase evenly, heat on a water bath at 40°C, ultrasonically emulsify, cool to 13°C, and obtain colostrum;
[0077] 4. According to the volume ratio of colostrum and polyvinyl alcohol solution is 1:10, add colostrum to 2% polyvinyl alcohol solution (containing the mass volume ratio of polyvinyl alcohol and normal saline 2g / 100ml, the same below), stir at high speed (6000r / min) for 2 minutes to obtain double emulsion;
[0078] 5. According to the volume ratio of double emulsion and normal saline as 1:50, put the double emulsion ...
Embodiment 2
[0083] 1. Weigh 600mg of liraglutide, add it to 120ml water for injection, and heat it on a water bath at 45°C to completely dissolve the drug as the inner water phase;
[0084] 2. Weigh 6 g of PLGA (75 / 25, molecular weight: 50,000 Daltons), add 130 ml of ethyl acetate to completely dissolve PLGA (75 / 25), and use it as the organic phase;
[0085] 3. Add the organic phase of step 2 to the inner water phase of step 1, mix the inner water phase and the organic phase evenly, heat on a water bath at 48°C, ultrasonically emulsify, cool to 12°C, and obtain colostrum;
[0086] 4. According to the volume ratio of colostrum and polyvinyl alcohol solution is 1:15, add colostrum into 1.5% polyvinyl alcohol solution that has been cooled to 12°C, stir at high speed (5500r / min) for 2 minutes to obtain double emulsion ;
[0087] 5. According to the volume ratio of double emulsion and normal saline is 1:100, put double emulsion into normal saline, heat in water bath at 30°C, stir at low speed...
Embodiment 3
[0092] 1. Weigh 500mg of liraglutide, add 100ml of water for injection, heat on a water bath at 45°C to completely dissolve the drug, and use it as the inner water phase;
[0093] 2. Weigh 5 g of PLGA (75 / 25, molecular weight: 50,000 Daltons), add 110 ml of dichloromethane to completely dissolve PLGA (75 / 25), and use it as the organic phase;
[0094] 3. Add the organic phase of step 2 to the inner water phase of step 1, mix the inner water phase and the organic phase evenly, heat on a water bath at 45°C, ultrasonically emulsify, cool to 14°C, and obtain colostrum;
[0095] 4. According to the volume ratio of colostrum and polyvinyl alcohol solution is 1:10, add colostrum into 2.5% polyvinyl alcohol solution that has been cooled to 14°C, stir at high speed (6500r / min) for 2 minutes to obtain double emulsion ;
[0096] 5. According to the volume ratio of double emulsion and normal saline is 1:50, put double emulsion into normal saline, heat in water bath at 30°C, stir at low sp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com