Spongy dextromethorphan hydrobromide film agent with micro-pore and preparation method thereof
A dextromethorphan hydrobromide and sponge-like technology, which can be used in medical preparations containing non-active ingredients, medical preparations containing active ingredients, and tablet delivery, etc., can solve the problem of increasing the proportion of plasticizers and slowing down dissolution , unable to achieve rapid effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
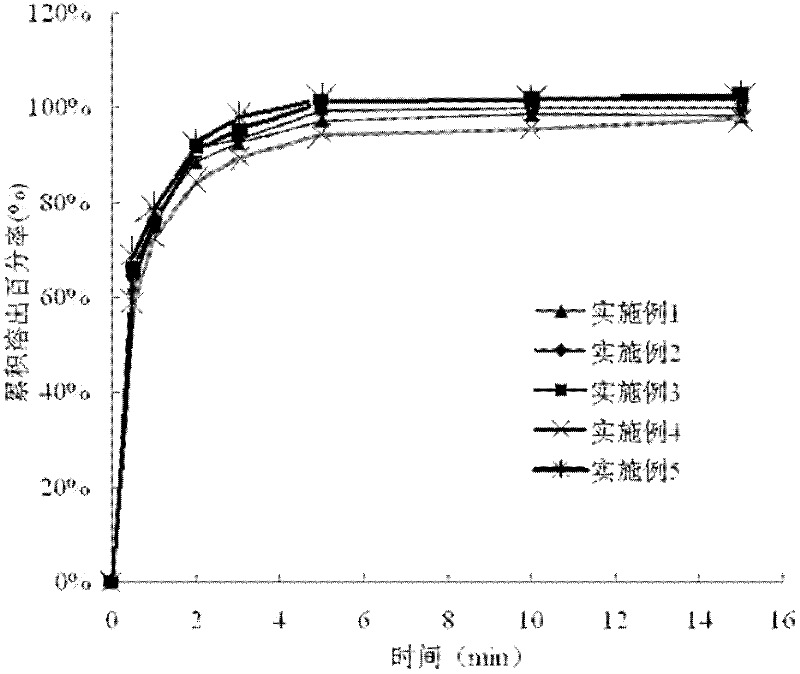
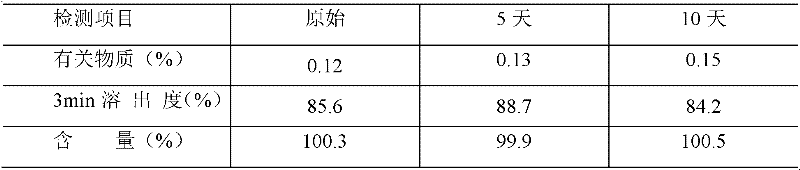
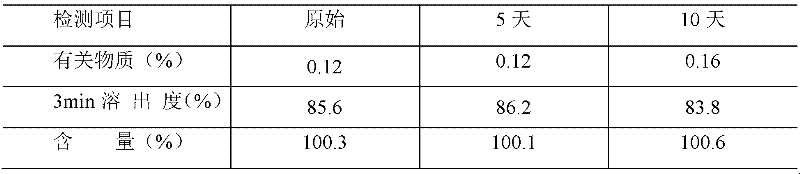
Examples
Embodiment 1
[0060] 80 g of PVA with a molecular weight of 130,000 Daltons and 20 g of HPC with a molecular weight of 600,000 Daltons were dissolved in 500 g of water to form a water slurry.
[0061] Add 100g of pore-forming agent, 10g of dextromethorphan hydrobromide, 2g of aspartame, 5g of glycerin and 3g of titanium dioxide into the above water slurry, fully stir and mix evenly.
[0062] The components of the pore-forming agent include chitin with a particle size of 0.5 μm and acetone adsorbed thereon, and the parts by weight of chitin and the parts by weight of the solvent are:
[0063] Chitosan 100 parts
[0064] Acetone 400 parts
[0065] (2) Then adopt the method reported in the literature of the research on nitroglycerin membrane (Chinese Journal of Pharmaceutical Industry, 1977, 12 (5)), apply and form a membrane, and obtain a thin film precursor;
[0066] (3) Dry the film precursor obtained in step (2) at 80° C. to obtain the film for the drug film preparation, with a thickness...
Embodiment 2
[0074] 16 g of HPMC with a molecular weight of 20,000 Daltons and 64 g of PEO with a molecular weight of 7,000,000 Daltons were dissolved in 500 g of water to form a water slurry.
[0075] Add 200g of pore-forming agent, 50g of dextromethorphan hydrobromide, 5g of sucralose and 0.1g of sunset yellow pigment into the above water slurry, fully stir and mix evenly.
[0076] The component of described pore-forming agent comprises the sodium polystyrene sulfonate of 10 μ m and the ethanol adsorbed on it, the parts by weight of sodium polystyrene sulfonate and the parts by weight of solvent are:
[0077] Sodium polystyrene sulfonate 100 parts
[0078] Ethanol 100 parts
[0079] Others are the same as embodiment 1.
[0080] The weight ratio of each component:
[0081] Low molecular weight water-soluble polymer material: high molecular weight water-soluble polymer material=1:4;
[0082] Water-soluble polymer material: water-insoluble micropowder = 1:1.25;
[0083] The diameter of...
Embodiment 3
[0086] Dissolve 60 g of sodium alginate with a molecular weight of 32,000 Daltons and 120 g of CMC-Na with a molecular weight of 700,000 Daltons in 700 g of water to form a water slurry.
[0087] Add 90g of pore-forming agent, 50g of dextromethorphan hydrobromide, 5g of cyclamate, 5g of PEG400 and 3g of titanium dioxide into the above water slurry, fully stir and mix evenly.
[0088] The components of the pore-forming agent include CaCO3 with a particle size of 1 μm and ethanol adsorbed thereon, and the parts by weight of CaCO3 and the parts by weight of the solvent are:
[0089] CaCO3 100 parts
[0090] Ethanol 100 parts
[0091] Others are the same as embodiment 1.
[0092] The weight ratio of each component:
[0093] Low molecular weight water-soluble polymer material: high molecular weight water-soluble polymer material=1:2;
[0094] Water-soluble polymer material: water-insoluble micropowder = 1:0.25;
[0095] The diameter of the micropores is 10 nm.
[0096] Perfor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com