1-aryl-2-acetone compound preparation method
A compound, aryl acrylic acid technology, applied in the field of 1-aryl-2-acetone compound synthesis, can solve the problems of high explosiveness, complicated operation, long steps, etc., and achieves reduction of reaction steps, improved operation safety, operation The effect of process coherence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
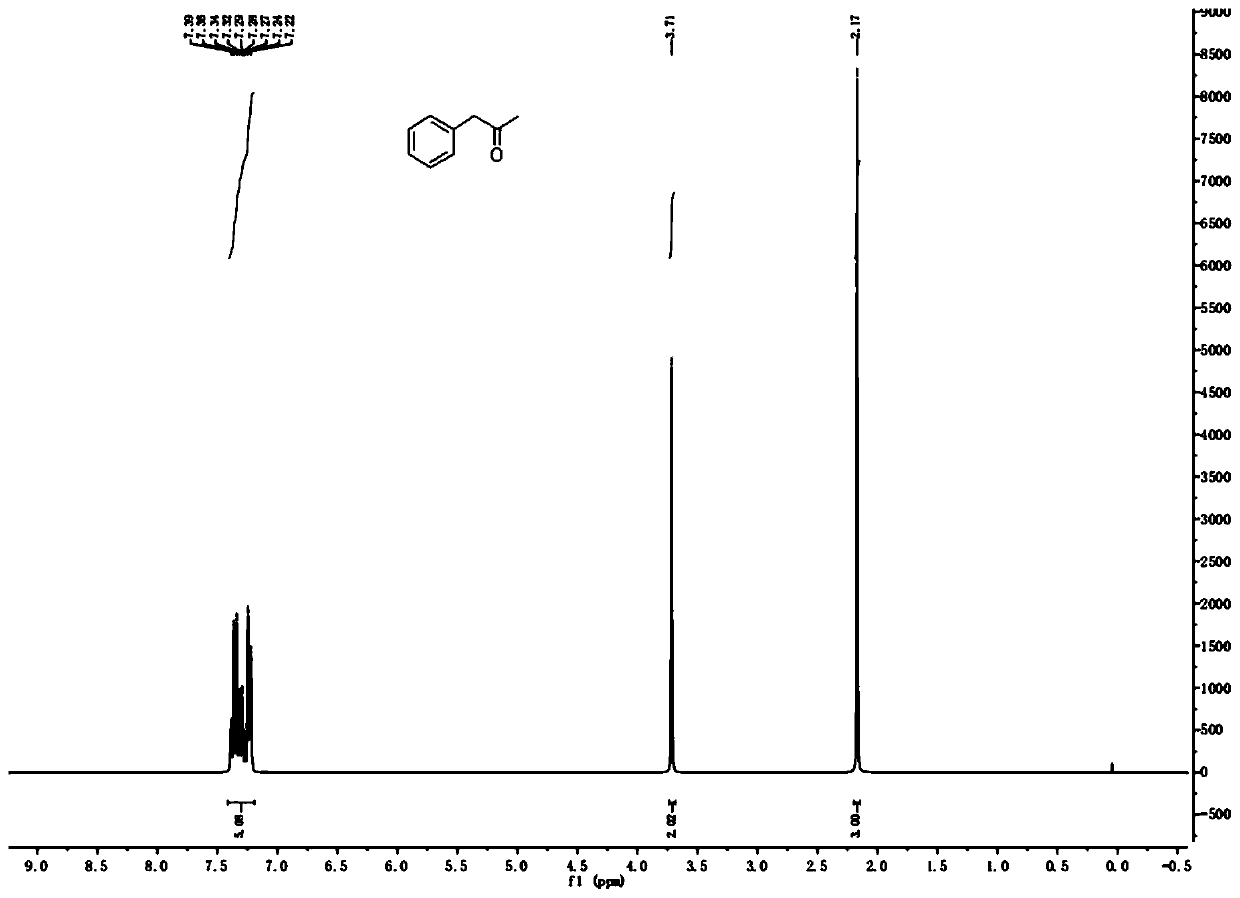
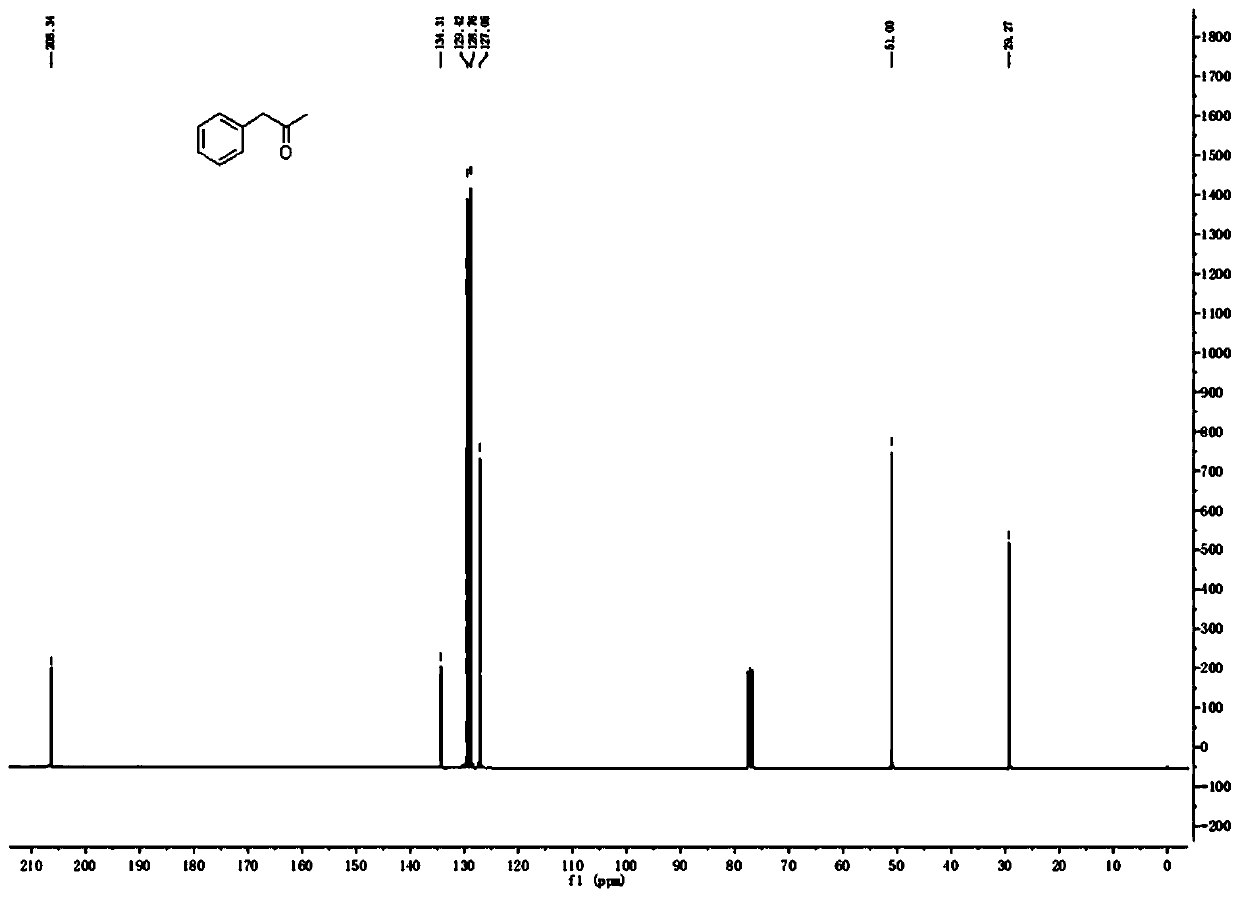
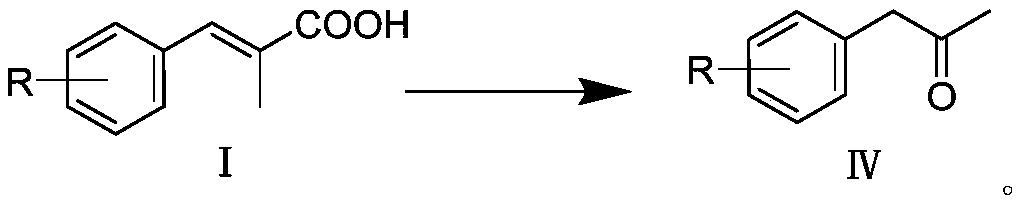
[0038] 30.0g (184.97mmol) of (E)-2-methyl-3-phenylacrylic acid, 200mL of toluene, 51.9g (188.67mmol) of DPPA and 19.6g (194.22mmol) of triethylamine were successively added to a 500mL reaction flask, The reaction was stirred at 25 °C for 30 min; the temperature was raised to 110 °C for 1 h; 100 mL of concentrated hydrochloric acid was added to the reaction solution, and the reaction was continued for 1 h. The reaction solution was cooled to room temperature, the organic layer was separated, the organic layer was washed with saturated sodium carbonate solution, and the toluene was evaporated under reduced pressure to obtain a yellow oily liquid. . The product is analyzed by gas chromatography, and the purity is over 99.5%. 1H NMR (CDCl 3, 300MHz): δ (ppm) 7.22-7.39 (m, 5H), 3.71 (s, 2H), 2.17 (s, 3H); 13C NMR (CDCl 3 , 101MHz): δ (ppm) 206.3, 134.3, 129.4, 128.8, 127.1, 51.0, 29.3; GC-MS (EI) found 134.2 [M + ].
Embodiment 2
[0040] (E)-2-methyl-3-(4-methoxyphenyl)acrylic acid 30.0 g (156.08 mmol), toluene 200 mL, DPPA 43.8 g (159.20 mmol) and triethylamine 16.6 g (163.88 mmol) were sequentially It was added to a 500 mL reaction flask, and the reaction was stirred at 25 °C for 30 min; the temperature was raised to 110 °C for 1.5 h; 100 mL of concentrated hydrochloric acid was added to the reaction solution, and the reaction was continued for 1 h. The reaction solution was cooled to room temperature, the organic layer was separated, the organic layer was washed with saturated sodium carbonate solution, and the toluene was evaporated under reduced pressure to obtain a yellow oily liquid. The crude product was rectified to obtain 1-(4-methoxyphenyl)-2-propanone 21.8g, 85% yield. The product is analyzed by gas chromatography, and the purity is over 99.5%. GC-MS(EI) found 164.1[M + ].
Embodiment 3
[0042] (E)-2-methyl-3-(3,4-dimethoxyphenyl)acrylic acid 30.0 g (134.99 mmol), toluene 200 mL, DPPA 37.9 g (137.69 mmol) and triethylamine 14.3 g (141.74 mmol) mmol) were sequentially added to a 500 mL reaction flask, and the reaction was stirred at 25 °C for 1 h; the temperature was raised to 110 °C for 1.5 h; 100 mL of concentrated hydrochloric acid was added to the reaction solution, and the reaction was continued for 1.5 h. The reaction solution was cooled to room temperature, the organic layer was separated, the organic layer was washed with saturated sodium carbonate solution, and the toluene was evaporated under reduced pressure to obtain a yellow oily liquid. The crude product was rectified to obtain 1-(4-methoxyphenyl)-2-propanone 21.5g, yield 82%. The product is analyzed by gas chromatography, and the purity is over 99.5%. GC-MS(EI)found 194.1[M + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com