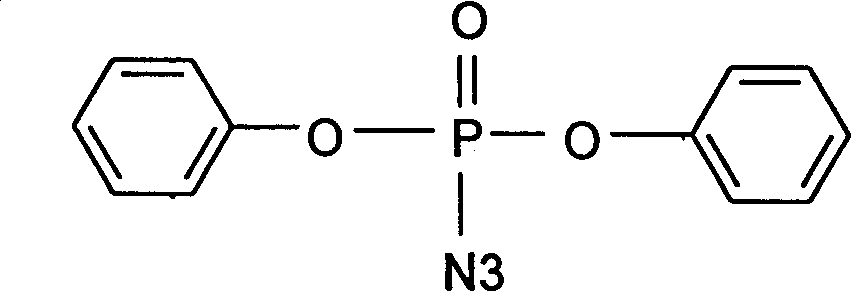
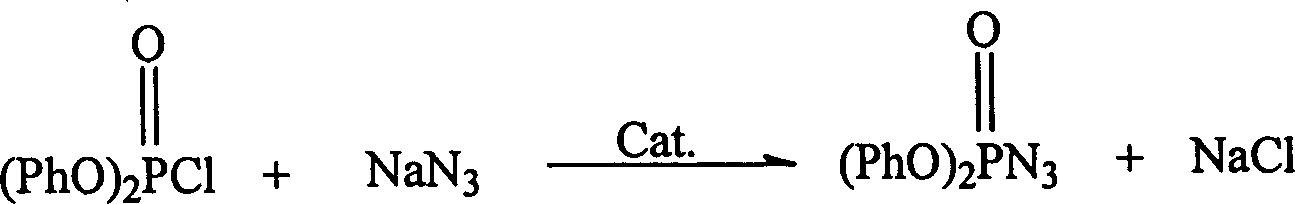
Catalystic preparation of nitrine diphenyl posphate
A technology of diphenyl azide phosphoric acid and diphenyl chlorophosphoric acid is applied in the field of preparing diphenyl phosphoric acid azide by using phase transfer catalyst catalysis, and can solve the problem that the reactant and the target product are difficult to be separated, and the yield and purity of the target product can be solved. It is not high, the preparation reaction time is long, etc., to achieve the effect of easy large-scale industrial preparation, short time and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 47.9g (0.178mol) of diphenyl chlorophosphate, 20.7g (0.32mol) of sodium azide, and 2.5g of tetrabutylammonium bromide into a 100ml three-necked flask and stir vigorously at room temperature for 10 hours. Viscous, then gradually become thinner, the color changes from colorless to light gray, and can be filtered to obtain 42.5g of slightly light yellow or colorless slightly viscous liquid, the yield is 86.2%, and the HPLC content is 99.3%.
[0021] Analysis data: IR(cm -1 ) 3060 (w, C-H), 2170 (s, -N3), 1590 (m), 1490 (s, C=C), 1270 (m, P=O), 960 (s, P-O-Aryl).
Embodiment 2
[0023] Add 48.1g (0.18mol) of diphenyl chlorophosphate, 27.6g (0.42mol) of sodium azide, and 3.0g of TEBA into a 100ml three-neck flask, stir vigorously at room temperature for 15 hours, and filter to obtain 41.8g of slightly light yellow liquid , HPLC content 97.7%, yield 85.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com