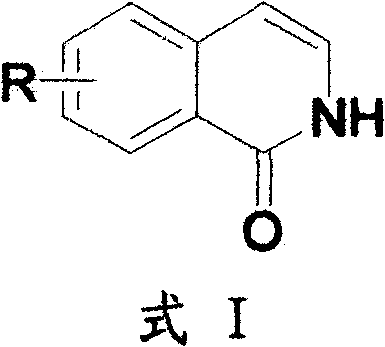
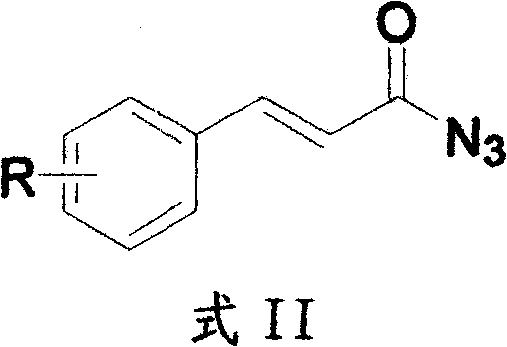
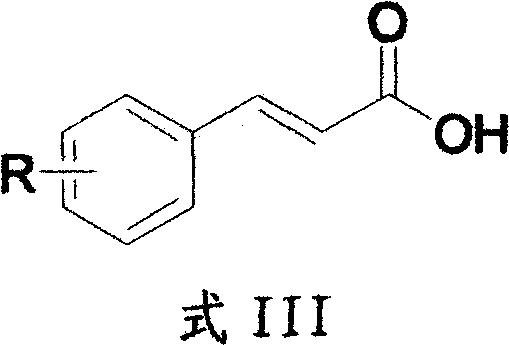
Method for preparing 2H-isoquinoline-1-ketones
A ketone compound and isoquinoline technology is applied in the field of preparation of 2H-isoquinoline-1-one compounds, and can solve the problems of easy spontaneous combustion of diisobutylaluminum hydride, expensive and unobtainable raw materials, harsh reaction conditions and the like , to achieve the effect of short reaction time, simple operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: Synthesis of 5-bromo-2H isoquinolin-1-one
[0024] 2-Bromocinnamic acid (41.7g, 0.183mol), triethylamine (7.44g, 73.64mmol) were dissolved in 300mL toluene and stirred at room temperature for 30 minutes. Diphenylphosphoryl azide DPPA (50.5 g, 0.138 mol) was added to the above reaction solution and the reaction was continued at room temperature for 2 hours, and the reaction of the raw materials was followed by thin layer chromatography (TLC). The reaction solution was diluted with 500 mL of ethyl acetate, and the organic phase was washed successively with 100 mL of 5% aqueous sodium hydroxide solution, 500 mL of water and 200 mL of saturated brine. The organic layer was dried with anhydrous sodium sulfate, and evaporated to dryness under reduced pressure by rotary evaporation to obtain 43.3 g of an azide intermediate with a yield of 95%. The above-mentioned azide intermediate (43.342g, 0.172mol) was dissolved in 200mL of diphenyl ether, and then 20.6mL of ...
Embodiment 2
[0026] Example 2: Synthesis of 5-trifluoromethoxy-2H isoquinolin-1-one
[0027] 2-Trifluoromethoxycinnamic acid (4.76 g, 20.50 mmol), triethylamine (2.07 g, 20.50 mmol) were dissolved in 50 mL of toluene and stirred at room temperature for 30 minutes. DPPA (5.63g, 20.50mmol) was added to the above reaction solution and the reaction was continued at room temperature for 2 hours, and the reaction of the raw materials was followed by TLC. The reaction solution was diluted with 100 mL of ethyl acetate, and the organic phase was washed successively with 20 mL of 5% aqueous sodium hydroxide solution, 50 mL of water and 20 mL of saturated brine. The organic layer was dried with anhydrous sodium sulfate, and evaporated to dryness under reduced pressure by rotary evaporation to obtain 4.8 g of an azide intermediate with a yield of 90%. The above-mentioned azide intermediate (4.7g, 18.27mmol) was dissolved in 30mL of diphenyl ether, and then 6mL of N,N-diisopropylethylamine was added d...
Embodiment 3
[0029] Example 3: Synthesis of 7-methoxy-2H isoquinolin-1-one
[0030] 4-Methoxycinnamic acid (5 g, 28.05 mmol), triethylamine (2.83 g, 28.05 mmol) were dissolved in 50 mL of toluene and stirred at room temperature for 30 minutes. DPPA (7.72g, 28.05mmol) was added to the above reaction solution, and the reaction was continued at room temperature for 2 hours, and TLC traced that the reaction of the raw materials was complete. The reaction solution was diluted with 100 mL of ethyl acetate, and the organic phase was washed successively with 20 mL of 5% aqueous sodium hydroxide solution, 50 mL of water and 20 mL of saturated brine. The organic layer was dried with anhydrous sodium sulfate, and evaporated to dryness under reduced pressure by rotary evaporation to obtain 5 g of azide intermediate with a yield of 90%. The above-mentioned azide intermediate (5.87g, 28.05mmol) was dissolved in 30mL of diphenyl ether, and then 14mL of N,N-diisopropylethylamine was added dropwise. After...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com