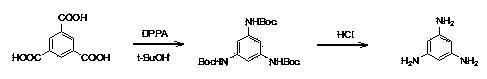
Synthesis process of 1, 3, 5-triaminobenzene
A technology of triaminobenzene and synthesis process, which is applied in the field of synthesis of 1,3,5-triaminobenzene, can solve the problems of high equipment requirements, dangerous transportation and use, and is not easy to enlarge, and achieves a simple synthesis route and convenient post-processing. , easy to magnify effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0014] The synthesis technique of 1,3,5-triaminobenzene, the steps are as follows:
[0015] Step 1: 1,3,5-Tri-Boc-aminobenzene
[0016] Diphenylphosphoryl azide (210 g, 764 mmol) was added in portions to trimesic acid (50 g, 238 mmol) and triethylamine (77.2 g, 764 mmol) in toluene (250 mL) and tert-butanol (150 mL) In the mixed solution, after the addition, the mixed solution is at 70 o C-90 o C stirred for 3-4 hours, then raised the temperature to 100 o C-120 o C and the reaction solution was stirred for 2 hours. After cooling down to room temperature, saturated sodium bicarbonate solution (400 mL) was added. The organic layer was separated and the aqueous phase was extracted twice with ethyl acetate. The combined organic phases were dried over anhydrous sodium sulfate and pulled dry. Petroleum ether (200 mL) was added to the residue, stirred, and then filtered to obtain 1,3,5-tri-Boc-aminobenzene (89 g, 90%). This solid was directly used in the next step without puri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com