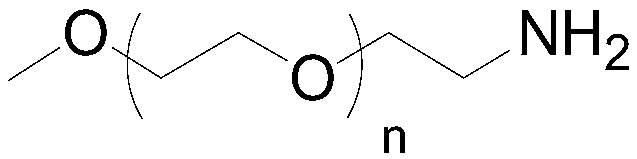
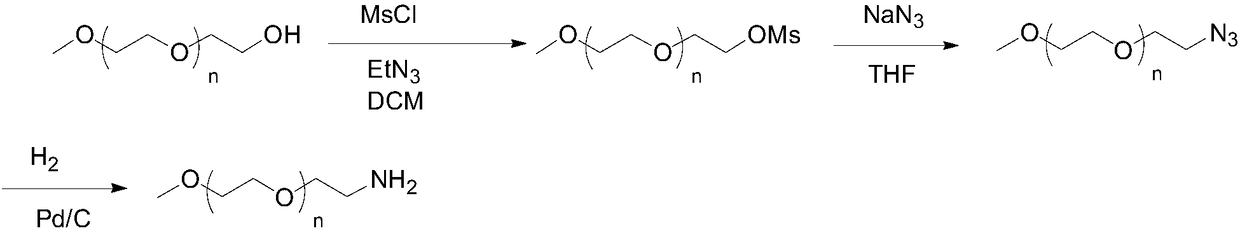
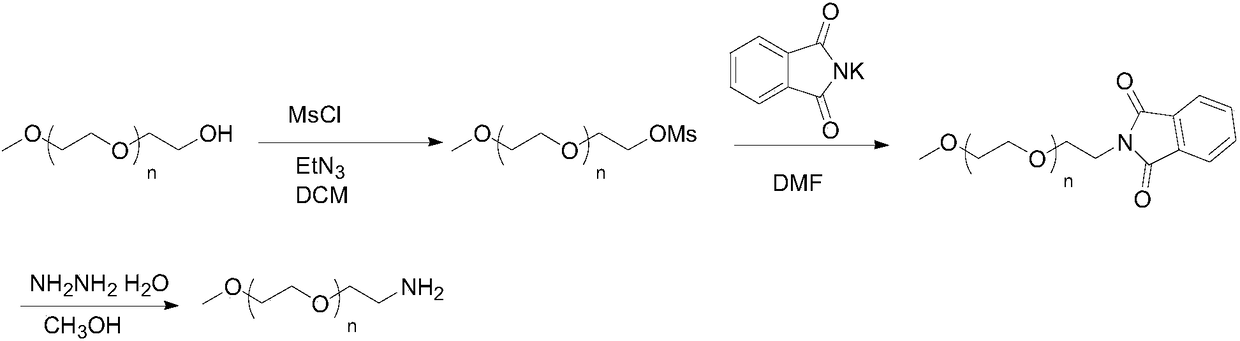
Preparation method of mPEG-amine
A technology of polyethylene glycol amine and monomethoxy, which is applied in the field of organic chemistry, can solve the problems of difficult industrial production and cumbersome operation, and achieve the effects of easy industrial production, simple post-processing and less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1.1 Preparation of compound monomethoxy polyethylene glycol azide 2000
[0039] Under the protection of nitrogen, 2.0 g of monomethoxy polyethylene glycol 2000 and 0.53 g of triphenylphosphine were added. Dissolve 0.56g of diphenylphosphoryl azide in 12mL of dry dichloromethane, place the reaction bottle in a low temperature environment of 0°C and stir for a certain period of time until the temperature in the reaction bottle is lower than 5°C, then slowly add azo 0.4 g of diisopropyl diformate, keep the temperature below 15°C during the dropwise addition. After the dropwise addition, the reaction system gradually changed from cloudy to clear, and the color changed from light yellow to golden yellow. After 3 hours of reaction, the reaction was complete, and the volume was evaporated to dryness under reduced pressure to obtain a yellow oil, which was purified by beating with anhydrous ether to obtain 1.72 g of the product with a yield of 84.13%.
[0040] 1H-NMR (CDCl 3...
Embodiment 2
[0067] 2.1 Preparation of compound monomethoxy polyethylene glycol amine 2000
[0068] Dissolve 2 g of monomethoxypolyethylene glycol azide 2000 in 20 mL of dry tetrahydrofuran, then add 0.2 g of 10% palladium carbon, replace the air in the reaction bottle with hydrogen, and react at 25°C for 24 hours, and the reaction is complete. The palladium carbon was removed by suction filtration, the solvent was evaporated to dryness under reduced pressure, and purified by beating with anhydrous diethyl ether to obtain 1.8 g of the product with a yield of 90.14%.
[0069] H-NMR (400M) δ = 3.65 (3H, s), 3.38 (93H, J = 8Hz, d)
[0070] MS(EI):m / e=2016.5
[0071] 2.2 Preparation of compound monomethoxy polyethylene glycol amine 2000
[0072] Dissolve 2 g of monomethoxypolyethylene glycol azide 2000 in 20 mL of dry tetrahydrofuran, then add 0.2 g of 5% palladium on carbon, replace the air in the reaction bottle with hydrogen, and react at 25°C for 24 hours, and the reaction is complete. ...
Embodiment 3
[0088] Preparation of Compound Monomethoxypolyethylene Glycol Azide 1000
[0089] Under the protection of nitrogen, 1.0 g of monomethoxy polyethylene glycol 1000 and 0.53 g of triphenylphosphine were added. Dissolve 0.56g of diphenylphosphoryl azide in 10mL of dry dichloromethane, place the reaction bottle in a low temperature environment of 0°C and stir for a certain period of time until the temperature in the reaction bottle is lower than 5°C, then slowly add azo 0.4 g of diisopropyl diformate, keep the temperature below 15°C during the dropwise addition. After the dropwise addition, the reaction system gradually changed from cloudy to clear, and the color changed from light yellow to golden yellow. After 3 hours of reaction, the reaction was complete, and the volume was evaporated to dryness under reduced pressure to obtain a yellow oil, which was purified by beating with anhydrous ether to obtain 0.86 g of the product with a yield of 83.25%.
[0090] 1H-NMR (CDCl3, 400M)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com