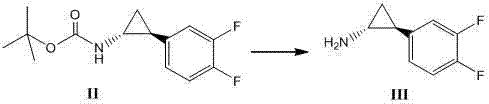
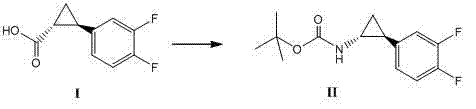
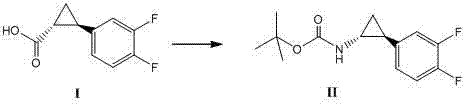
Method for synthesizing trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine
A technology of difluorophenyl and cyclopropylamine, which is applied in the preparation of amino compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problem of increasing the difficulty of the reaction process, the difficulty of preparation and the increase in cost, and is not suitable for large-scale industrialization. Production and other problems, to achieve the effect of improving the reaction yield and reducing the difficulty of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Synthetic method of trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine
[0030] .
[0031] In a 500ml three-necked flask, add 50.0g (252.3mmol) of the formula under nitrogen protection The shown compound [made according to CN1431992A Example 9], 76.4g (277.54mmol) diphenylphosphoryl azide (DPPA), 25.5g (253.3mmol) triethylamine (Et 3 N), 250ml tert-butanol (t-BuOH), stirred and dissolved, heated to 85°C and reacted for 2 hours, TLC (EA:PE=1:10) showed that the raw material disappeared, concentrated under reduced pressure, removed the solvent, and purified by column chromatography to obtain 54.0 g of white solid, 79.5% yield.
[0032] 1 HNMR (CDCl 3 , 400Mhz) δ:7.07~6.90(3H,m),4.84(1H,s),2.65~2.64(1H,m),2.03~1.99(1H,m),1.45(9H,s),1.15~1.10( 2H,m).
[0033] Ms (API-ES): 270 (M+1).
[0034]
[0035] In a 500ml three-necked flask, add 50.0g (185.7mmol) of formula under nitrogen protection The compound shown, 200ml of 2N hydrochloric acid / ethyl ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com