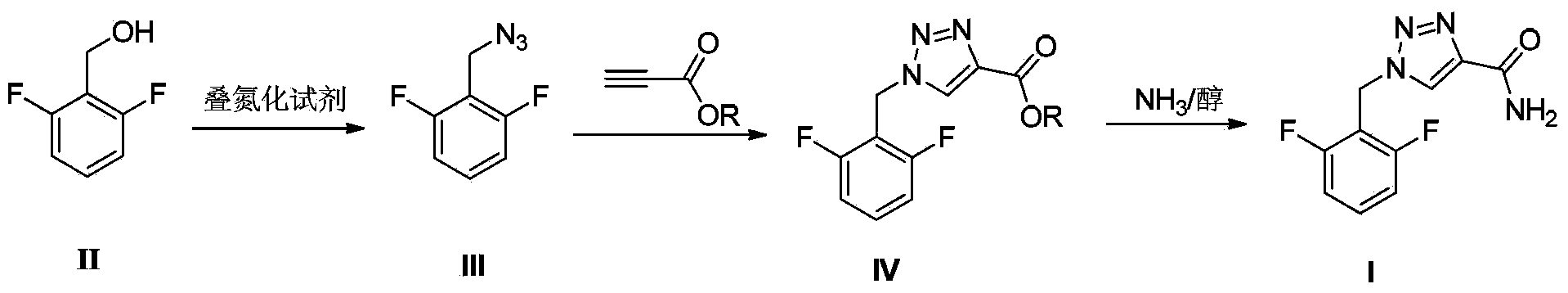
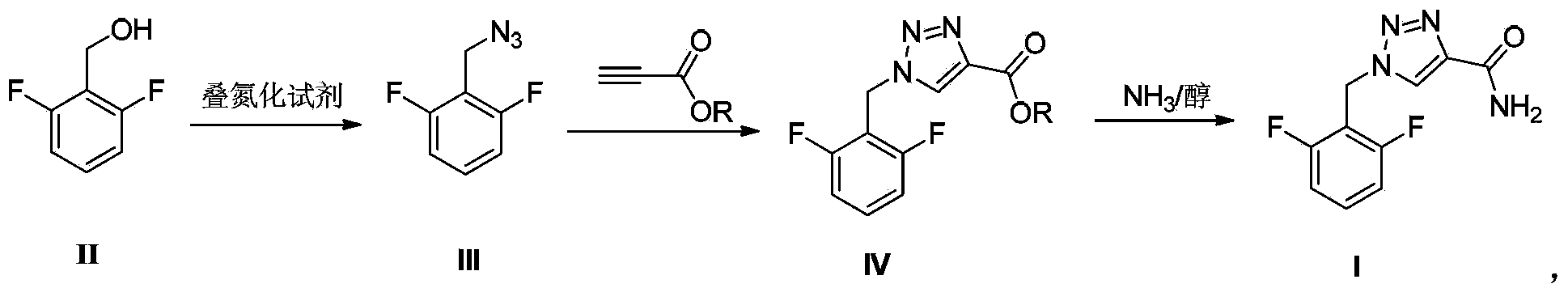
A rufinamide preparing method
A technology of rufinamide and reaction, which is applied in the field of preparation of rufinamide, can solve problems such as long production cycle, strong corrosion, and environmental pollution, and achieve the effects of shortened reaction time, shortened production cycle, and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Preparation of 2,6-difluorobenzyl azide (III)
[0023] Add 2,6-difluorobenzyl alcohol (72.06g, content 98%, 0.50mol), diphenylphosphoryl azide (151.36g, 0.55mol) and toluene (600mL) into a 1L three-necked flask. Heated to reflux with stirring and reacted for 6 hours. Add 200 mL of purified water, and adjust the pH to 12 with sodium hydroxide while stirring. Liquid extraction was carried out, and the aqueous layer was extracted once with 100 mL of toluene. The organic layers were combined, washed with saturated brine, and dried over anhydrous magnesium sulfate (100 g). After filtration, the filtrate was concentrated under reduced pressure, and toluene was recovered to obtain 77.81 g of oily liquid with a yield of 92.0%. ESI-MS(m / z): 170.1[M+H] + ; HPLC purity: 97.5%.
Embodiment 2
[0024] Example 2 Preparation of 2,6-difluorobenzyl azide (III)
[0025] Add 2,6-difluorobenzyl alcohol (72.06g, content 98%, 0.50mol), diphenylphosphoryl azide (275.2g, 1.0mol) and toluene (600mL) into a 1L three-necked flask. Heated to reflux with stirring and reacted for 6 hours. Add 200 mL of purified water, and adjust the pH to 12 with sodium hydroxide while stirring. Liquid extraction was carried out, and the aqueous layer was extracted once with 100 mL of toluene. The organic layers were combined, washed with saturated brine, and dried over anhydrous magnesium sulfate (100 g). After filtration, the filtrate was concentrated under reduced pressure, and the toluene was recovered to obtain 78.35 g of oily liquid with a yield of 93.0%. ESI-MS(m / z): 170.1[M+H] + ; HPLC purity: 97.5%.
Embodiment 3
[0026] Example 3 Preparation of ethyl 1-(2,6-difluorophenyl)-1H-1,2,3-triazole-4-carboxylate
[0027] Add the product of Example 1 (76.11g, 0.45mol) into a 500mL three-neck flask, add 300mL of absolute ethanol, and dissolve completely under stirring. Ethyl propiolate (88.29 g, 0.90 mol) was added, and the mixture was heated under reflux for 4 hours. After cooling down to room temperature, a solid precipitated out. After filtering, the filter cake was washed with absolute ethanol to obtain 106.89 g of light yellow solid with a yield of 88.9%. ESI-MS(m / z): 268.0[M+H] + ; HPLC purity: 97.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com