Asymmetric alcoholysis catalyzed by chiral thiourea amine salt
An asymmetric, thiourea ammonium salt technology, applied in the preparation of organic compounds, organic chemistry, organic chemical methods and other directions, can solve the problems of low yield, need to split, long synthesis route of pregabalin, etc., and achieves few steps. , mild reaction conditions, novel synthetic route effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1. Catalytic asymmetric alcoholysis
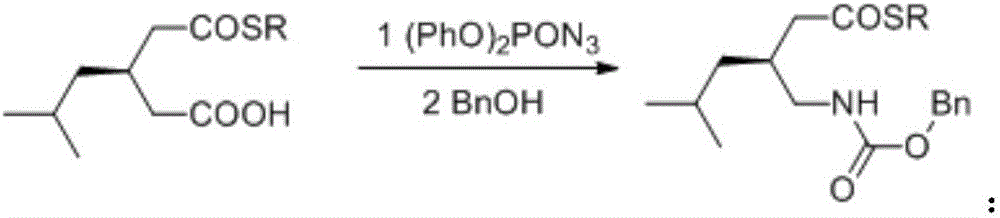
[0039] Take a 100mL three-necked flask, add 3-isobutylglutaric anhydride (1.70g, 0.01mol), bromide (S)-2-(3(3,5-bis(trifluoro Methyl)phenyl)thioureido)-N,N,4-trimethyl-N-(4-bromobenzyl)pentan-1-ammonium (665mg, 1.0mmol), and 50 mL THF was added to dissolve the solid, Benzylthiol (1.50g, 0.012mol) was added under stirring at -10°C, reacted for 16h, the solvent was removed under reduced pressure, and purified by column chromatography to obtain 2.80g of thiol ester with a yield of 95% and an ee value of 94%. 1 H NMR (400MHz, CDCl 3 ): δ=0.80-0.87(6H), 1.13(2H), 1.55(1H), 2.25-2.40(3H), 2.50-2.60(2H), 4.34(2H), 7.26-7.42(5H), 10.70(1H ) ppm; MS (ESI) calcd for C 16 h 22 o 3 S(M + ):294.4; Found:295.2(M+H) + .
[0040] 2. Synthesis of benzyloxycarbamide methyl thiol ester
[0041] Take a 100mL three-necked flask, dissolve thiol ester (2.80g, 9.5mmol) in dry toluene at 25°C, add triethylamine (0.97g, 9.6mmol) and diphenylphosphor...
Embodiment 2
[0047] 1. Catalytic asymmetric alcoholysis
[0048] Take a 100mL three-necked flask, add 3-isobutylglutaric anhydride (1.70g, 0.01mol), chloride (S)-2-(3(3,5-bis(trifluoro Methyl)phenyl)thioureido)-N,N,4-trimethyl-N-(4-bromobenzyl)pent-1-ammonium (620mg, 1.0mmol), and added 50mL methyl tert-butyl Dissolve the solid in ether, add benzylmercaptan (1.50g, 0.012mol) under stirring at -10°C, react for 16h, remove the solvent under reduced pressure, and purify by column chromatography to obtain 2.84g of thiol ester with a yield of 96% and an ee value of 95 %. 1 H NMR (400MHz, CDCl 3 ): δ=0.80-0.87(6H), 1.10(2H), 1.55(1H), 2.25-2.40(3H), 2.50-2.60(2H), 4.34(2H), 7.26-7.40(5H), 10.70(1H ) ppm; MS (ESI) calcd for C 16 h 22 o 3 S(M + ):294.4; Found:295.2(M+H) + .
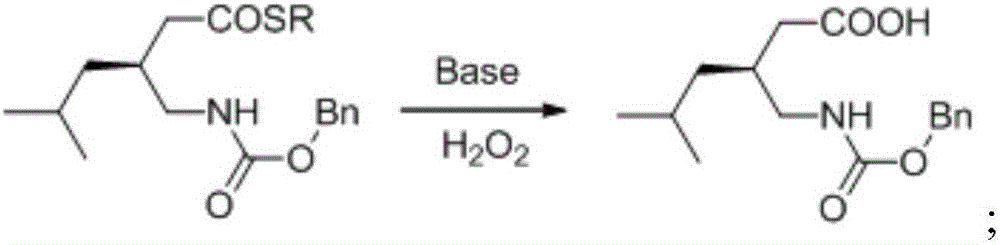
[0049] 2. Synthesis of benzyloxycarbamide methyl thiol ester
[0050] Take a 50mL three-necked flask, dissolve thiol ester (1.42g, 4.8mmol) in dry toluene at 25°C, add triethylamine (0.49g, 4.8mmol) and diphenylphos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com