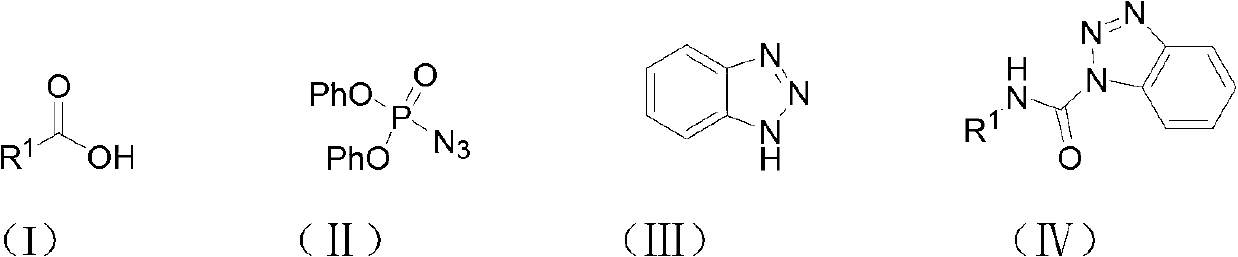
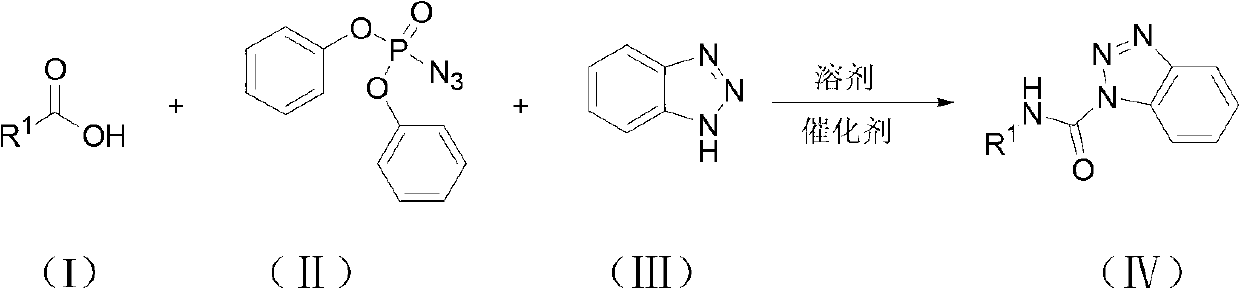
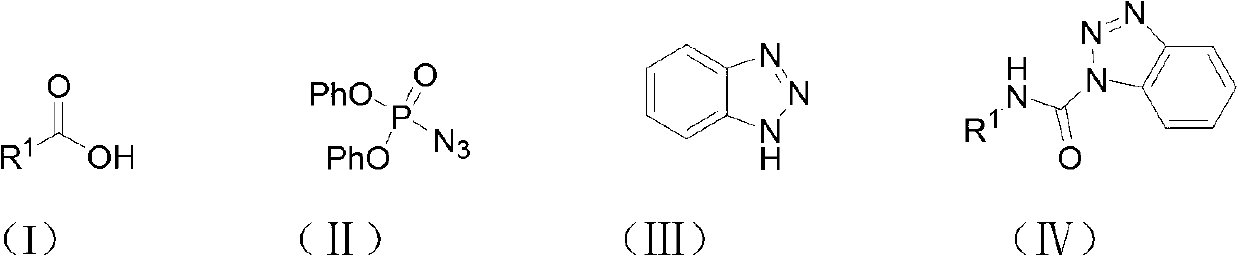
Method for synthesizing carbamyl benzotriazole by three-component one-pot method
A technology of carbamoylbenzotriazole and benzotriazole, which is applied in the field of three-component one-pot synthesis of carbamoylbenzotriazole, which can solve the problems of difficult storage and difficult application in industrial production, and achieve high yield High, mild reaction conditions, high implementation value and potential socio-economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the preparation of N-phenylcarbamoylbenzotriazole
[0019] The amount ratio of feed material is benzoic acid: diphenylphosphoric azide: benzotriazole: catalyst=1.0: 1.0: 1.2: 2.0, wherein the consumption of benzoic acid is 10mmol, organic solvent is toluene, and its consumption is the quality of benzoic acid 20 times of that, the catalyst is triethylamine, and the reflux time is 6 hours.
[0020] In a 100mL round bottom flask equipped with a reflux condenser, add 1.22g (10mmol) of benzoic acid, 2.75g (10mmol) of diphenylphosphoryl azide, 1.43g (12mmol) of benzotriazole, and 2.02g of triethylamine ( 20mmol), toluene 24.4g, stirred under reflux for 6 hours, followed by TLC until the reaction of the raw materials was complete, after the reaction was complete, the solvent was removed under reduced pressure, dissolved in ethyl acetate, washed once with saturated sodium bicarbonate solution, and washed once with saturated sodium chloride solution , the organic ...
Embodiment 2
[0021] Embodiment 2: Preparation of N-phenylcarbamoylbenzotriazole
[0022] The amount ratio of the feed material is benzoic acid: diphenylphosphoric azide: benzotriazole: catalyst=1.0: 2.0: 1.2: 3.0, wherein the consumption of benzoic acid is 10mmol, the organic solvent is toluene, and the consumption is the quality of benzoic acid 30 times of that, the catalyst is triethylamine, and the reflux time is 4 hours.
[0023] Others are the same as in Example 1, the product is N-phenylcarbamoylbenzotriazole solid, and 1.55 g of the product is obtained, with a yield of 65% and a purity of 97.1%.
Embodiment 3
[0024] Embodiment 3: the preparation of N-phenylcarbamoylbenzotriazole
[0025] The amount ratio of the feed material is benzoic acid: diphenylphosphoric azide: benzotriazole: catalyst=1.0: 2.5: 1.2: 2.5, wherein the consumption of benzoic acid is 10mmol, the organic solvent is toluene, and the consumption is the quality of benzoic acid 20 times of that, the catalyst is triisopropylamine, and the reflux time is 5 hours.
[0026] Others are the same as in Example 1, the product is N-phenylcarbamoylbenzotriazole solid, and 1.50 g of the product is obtained, with a yield of 63% and a purity of 98.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com