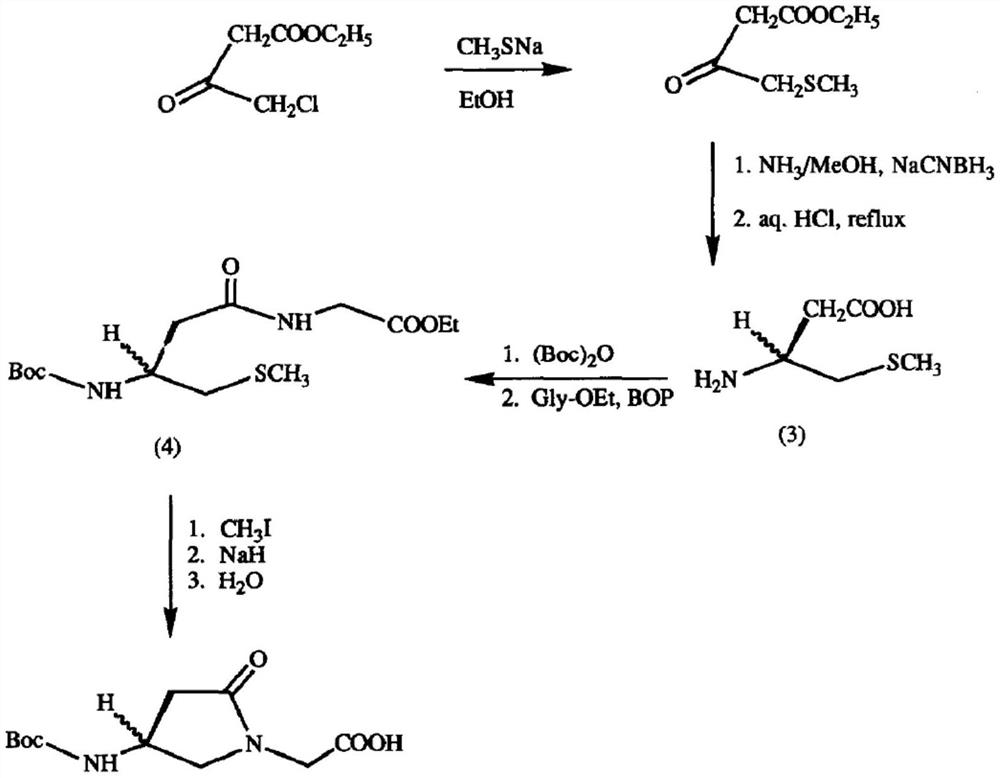
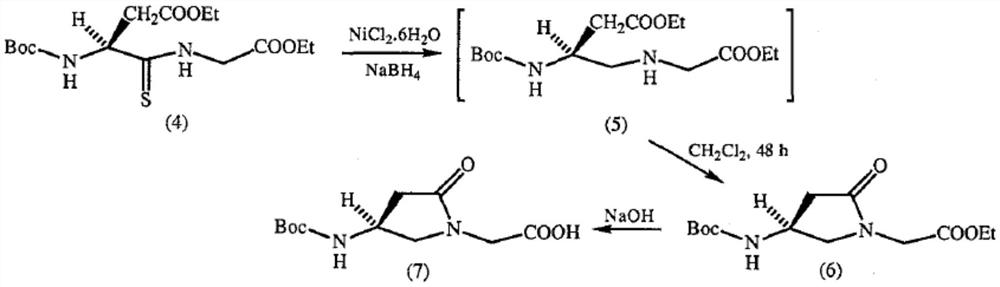
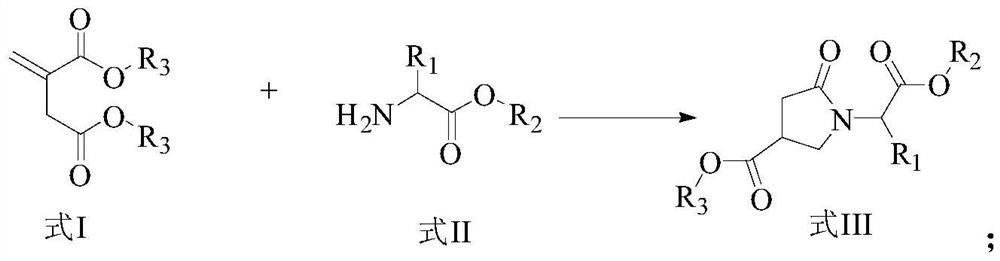
A kind of preparation method of γ-lactam bridged dipeptide compound
A technology for lactam bridges and compounds, which is applied in the field of preparation of γ-lactam bridged dipeptide compounds, can solve the problems of low yield in the cyclization step and decrease in the total yield of the route, and achieve high product yield and benefit Environmental protection and simple synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] In this example, the preparation process of 2-{4-[(tert-butoxy)carbonylamino]-2-oxopyrrolidinyl}acetic acid is as follows:
[0057]
[0058] The preparation method comprises the following steps:
[0059] Synthesis of tert-butyl 2-[4-(methoxycarbonyl)-2-oxopyrrolidinyl]acetate (3'):
[0060] Add 100ml of methanol, compound 1' (25g, 0.16mol, 1.0eq) and compound 2' (25g, 0.19mol, 1.2eq) into a 250ml three-necked flask, and reflux for 16h under nitrogen protection. The reaction is basically complete by TLC, spin-dried, Low-boiling impurities were distilled off under reduced pressure, and the residue was distilled to obtain compound 3' (38 g, yield 93%). MS-EI = 257.1. 1 H NMR (300MHz, CDCl 3 ): δ4.09-3.83 (m, 2H), 3.75-3.66 (m, 5H), 3.36-3.25 (m, 1H), 2.84-2.64 (m, 2H), 1.47 (s, 9H).
[0061] Synthesis of 1-{[(tert-butyl)oxycarbonyl]methyl}-5-oxopyrrolidine-3-carboxylic acid (4'):
[0062] Add 200ml of tetrahydrofuran and compound 3' (38g, 0.15mol, 1eq) into a 500ml...
Embodiment 2
[0068] In this example, the preparation process of (S)-2-methyl-2-{4-[(tert-butoxy)carbonylamino]-2-oxopyrrolidinyl}acetic acid is as follows:
[0069]
[0070] The preparation method comprises the following steps:
[0071] Synthesis of tert-butyl (S)-2-methyl-2-[4-(methoxycarbonyl)-2-oxopyrrolidinyl]acetate (8):
[0072] Add 100ml of methanol, compound 1' (22.7g, 0.14mol, 1.0eq) and compound 7 (24g, 0.168mol, 1.2eq) into a 250ml three-necked flask, and reflux for 16h under nitrogen protection. The reaction is basically complete by TLC, spin-dried, Low-boiling impurities were distilled under reduced pressure, and the residue was distilled to obtain compound 8 (28 g, yield 75%). MS-EI = 271.1. 1 H NMR (300MHz, CDCl3): δ4.82-4.72(m, 1H), 3.81-3.61(m, 5H), 3.36-3.19(m, 1H), 2.84-2.63(m, 2H), 1.46(s, 9H), 1.42-1.37 (m, 3H).
[0073] Synthesis of (S)-1-{1-[(tert-butyl)oxycarbonyl]ethyl}-5-oxopyrrolidine-3-carboxylic acid (9):
[0074] Add 200ml of tetrahydrofuran and compou...
Embodiment 3
[0080] In this example, the preparation process of (S)-2-(2-methylpropyl)-2-{4-[(tert-butoxy)carbonylamino]-2-oxopyrrolidinyl}acetic acid is as follows :
[0081]
[0082] The preparation method comprises the following steps:
[0083] Synthesis of tert-butyl (S)-2-(2-methylpropyl)-2-[4-(methoxycarbonyl)-2-oxopyrrolidinyl]acetate (13):
[0084] Add 100ml of methanol, compound 1' (16.8g, 0.11mol, 1.0eq), compound 12 (31.32g, 1.5eq, 0.165mol) into a 250ml three-necked flask, and reflux for 16h under nitrogen protection. The reaction is basically complete by TLC, and spin-dried , Low-boiling impurities were distilled off under reduced pressure, and the residue was distilled to obtain compound 13 (28.8 g, yield 82%). MS-EI=313. 1H NMR (300MHz, CDCl3): δ4.69(t, 1H), 3.83-3.67(m, 4H), 3.51(t, 1H), 3.24-3.12(m, 1H), 2.78-2.57(m, 2H) , 1.70-1.56 (m, 2H), 1.41 (s, 10H), 0.93-0.88 (m, 6H).
[0085] Synthesis of (S)-1-{1-[(tert-butyl)oxycarbonyl]-3-methylpropyl}-5-oxopyrrolidine-3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com